Book traversal links for 1266
A new class of low-complexity manual NAATs (LC-mNAATs) has now emerged for alternative molecular solutions that have improved accuracy when compared with smear microscopy and very basic infrastructure, power and equipment requirements (e.g. heat block). LC-mNAATs can be performed at the microscopy level and are currently cheaper than other molecular tests. Collectively, these characteristics are useful for testing in constrained settings. However, like smear microscopy, this class of tests does not incorporate rifampicin-resistance detection and therefore requires reflex testing with a complementary solution for drug-resistance determination.
2.2.1 Low-Complexity manual NAATs for detection of TBNEW
Diagnostic class description
The features shown in Table 2.2.1.1 define the class of LC-mNAATs.
Table 2.2.1.1 Class criteria for LC-mNAATs
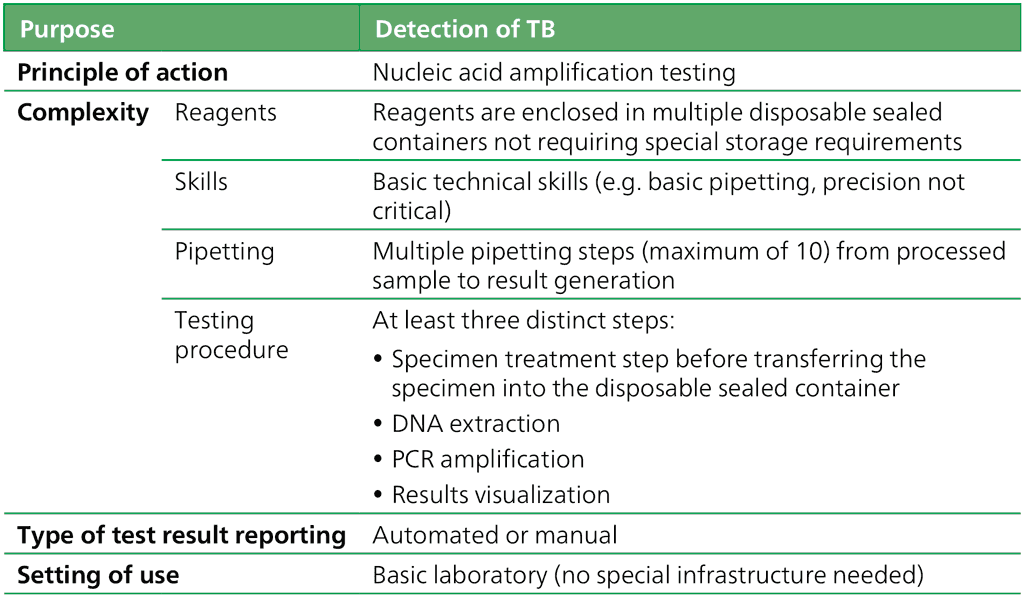
DNA: deoxyribonucleic acid; LC-mNAAT: low-complexity manual nucleic acid amplification test; PCR: polymerase chain reaction; TB: tuberculosis.
The only product for which eligible data met the class-based performance criteria for LC-mNAATs is Loopamp MTBC Detection Kit (TB LAMP) (Eiken Chemical, Tokyo, Japan) for pulmonary TB.
Regulatory approval from national regulatory authorities or other relevant bodies is required before implementation of this diagnostic test. Extrapolation to other brand-specific tests cannot be made, and any new in-class technologies or new indications for the technology currently included in the class will need to be evaluated by WHO PQ and WHO/GTB, respectively.
The publication WHO operational handbook on tuberculosis. Module 3: Diagnosis describes the tests included in this class.
Recommendations
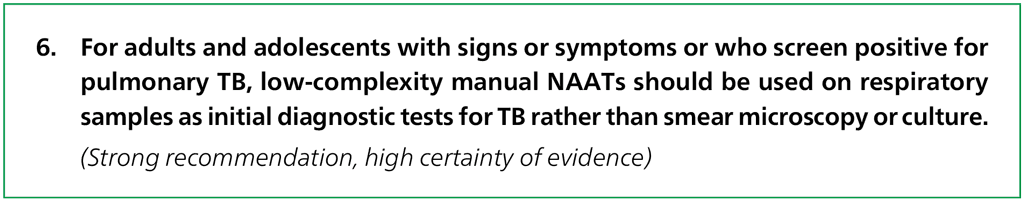
Remarks
- This recommendation applies to all people living with HIV, with the caveat of low to moderate certainty of evidence. However, wherever available, concurrent testing with an LC-aNAAT and LF-LAM is recommended for people living with HIV. For more details, see Section 2.3.1.
- This recommendation was extrapolated to children for use with respiratory samples (including induced sputum and gastric aspirate) based on the generalization of data from adults and very limited data for children, acknowledging the difficulties of collecting sputum specimens from this population. However, wherever available, concurrent testing with an LC-aNAAT on a respiratory and stool samples is recommended for children. For more details, see Section 2.3.2.
- Data on the use of the test with paediatric stool samples were very limited, and there were no data on the use of nasopharyngeal aspirates. The recommendation was, therefore, not extrapolated to these sample types.
- No recommendation was made on test use for extrapulmonary TB due to insufficient data.
- As LC-mNAATs do not provide rifampicin-resistance results, all positive diagnostic tests for TB require follow-up and referral for DST for, at a minimum, rifampicin.
Justification and evidence
WHO/GTB initiated an update of the previous guidelines and commissioned a systematic review on the use of LC-mNAATs (TB LAMP) for the diagnosis of TB in people with signs and symptoms of TB, or who screened positive for TB.
Detection of pulmonary TB
Should LC-mNAATs on respiratory samples be used to diagnose pulmonary TB in adults and adolescents with signs and symptoms or who screened positive for pulmonary TB, against an MRS?
Twenty-six studies (18 297 participants) assessed diagnostic accuracy using sputum specimens and comparing with an MRS. The sensitivities were between 55% and 100%, and the specificities were between 70% and 100% (Fig. 2.2.1.1). The summary sensitivity was 84.1% (95% CI: 78.3–88.6), and the summary specificity was 96.1% (95% CI: 94.2–97.4). The certainty of evidence for sensitivity and specificity was high.
Fig. 2.2.1.1 Forest plot of LC-mNAAT sensitivity and specificity for detection of pulmonary TB in respiratory samples and MRSᵃ
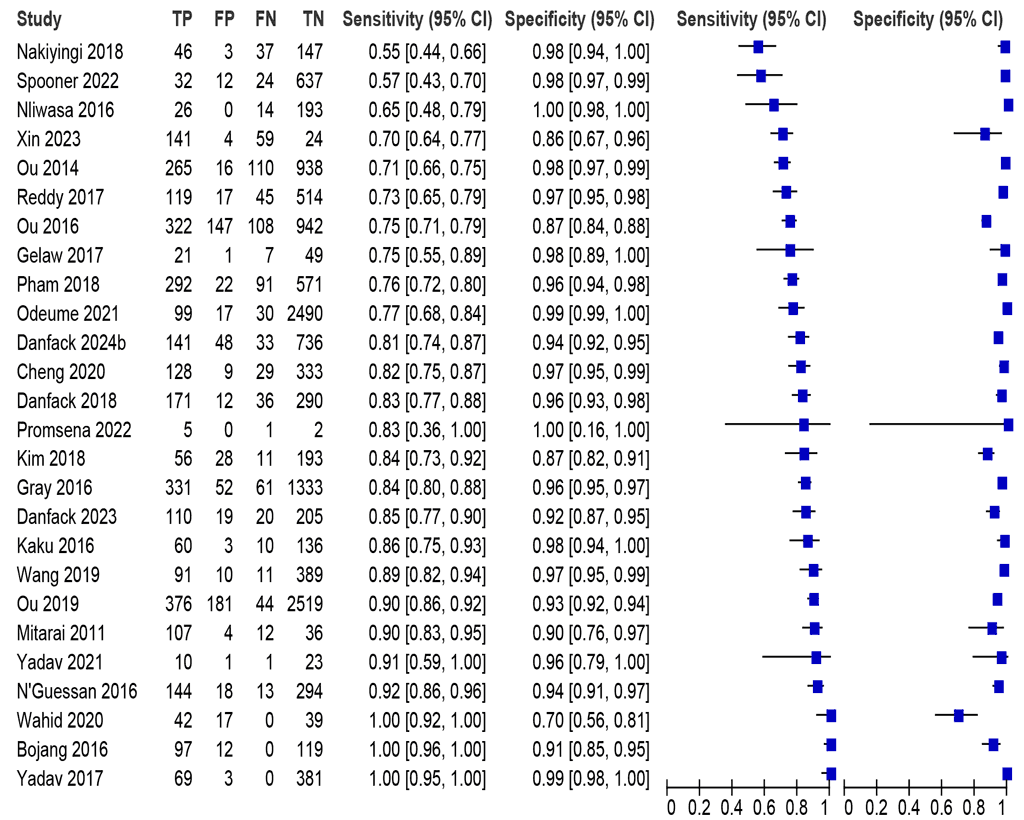
CI: confidence interval; FN: false negative; FP: false positive; LC-mNAAT: low-complexity manual nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
a Studies are sorted by increasing sensitivity.
Detection of TB in people living with HIV
Should LC-mNAATs on respiratory samples be used to diagnose pulmonary TB in adult and adolescent living with HIV with signs and symptoms of pulmonary TB, against an MRS?
In the eight studies (2991 participants) included in this meta-analysis, the sensitivities ranged between 52% and 100%, and the specificities between 27% and 100% (Fig. 2.2.1.2). The summary sensitivity was 77.1% (95% CI: 60.8–87.9), and the summary specificity was 95.9% (95% CI: 84.9–99.0). The certainty of evidence was low for sensitivity and moderate for specificity.
Fig. 2.2.1.2 Forest plot of LC-mNAAT sensitivity and specificity for detection of pulmonary TB in respiratory samples from people living with HIV and MRSᵃ
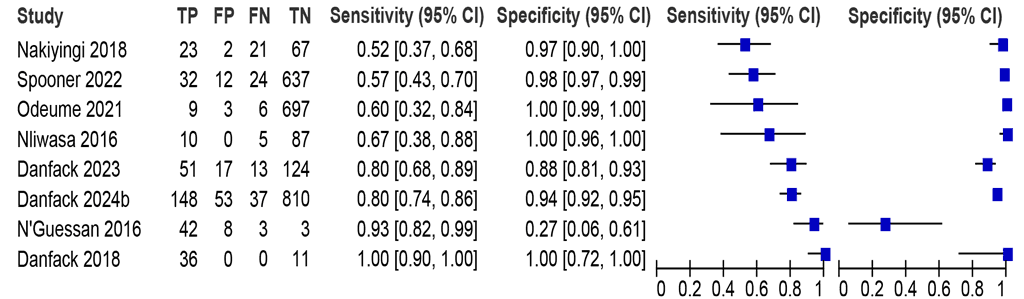
CI: confidence interval; FN: false negative; FP: false positive; HIV: human immunodeficiency virus; LC-mNAAT: lowcomplexity manual nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
a Studies are sorted by increasing sensitivity.
Detection of TB in children
Should LC-mNAATs on respiratory samples be used to diagnose pulmonary TB in children with signs and symptoms of pulmonary TB, against an MRS?
Three studies (62 participants, including eight with pulmonary TB) assessed the accuracy of LC-mNAATs for detecting pulmonary TB using respiratory samples (sputum, BAL and tracheal aspirate) and an MRS (Fig. 2.2.1.3). The sensitivities were between 60% and 100%, and the specificities were between 95% and 100%. The certainty of evidence was very low for sensitivity and low for specificity.
Fig. 2.2.1.3 Forest plot of LC-mNAAT sensitivity and specificity for detection of pulmonary TB in respiratory samples and MRSᵃ
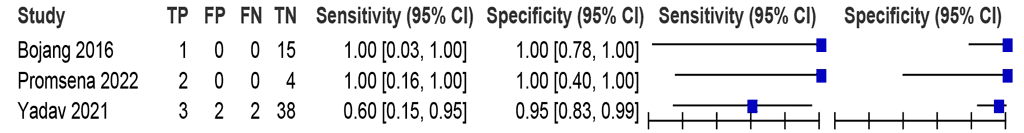
CI: confidence interval; FN: false negative; FP: false positive; LC-mNAAT: low-complexity manual nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
a Studies are sorted by increasing sensitivity.
Should LC-mNAATs on gastric aspirate be used to diagnose pulmonary TB in children with signs and symptoms of pulmonary TB, against an MRS?
Three studies (176 participants, including 14 with pulmonary TB) assessed the accuracy of LC-mNAATs for detecting pulmonary TB using gastric aspirate against a MRS (Fig. 2.2.1.4). Sensitivity was not estimable for two studies and was 64% in the third study. The specificities were between 93% and 100%.
Fig. 2.2.1.4 Forest plot of LC-mNAAT sensitivity and specificity for detection of pulmonary TB in gastric aspirate and MRSᵃ
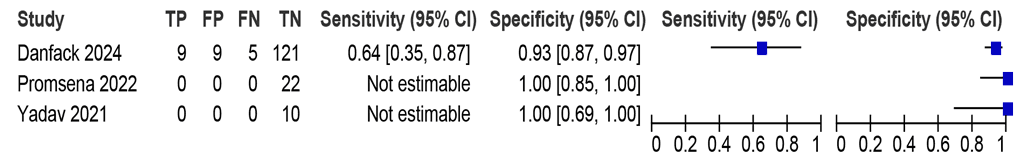
CI: confidence interval; FN: false negative; FP: false positive; LC-mNAAT: low-complexity manual nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
a Studies are sorted by increasing sensitivity.
Should LC-mNAATs on nasopharyngeal aspirate be used to diagnose pulmonary TB in children with signs and symptoms of pulmonary TB, against an MRS?
One study (144 participants including 12 with pulmonary TB) assessed the accuracy of LC-mNAATs for detecting pulmonary TB using nasopharyngeal aspirate against an MRS (Fig. 2.2.1.5). The sensitivity was 58% and specificity was 94%. Due to limited data, a recommendation on using LC-mNAATs with nasopharyngeal aspirate for detection of pulmonary TB was not made.
Fig. 2.2.1.5 Forest plot of LC-mNAAT sensitivity and specificity for detection of pulmonary TB in nasopharyngeal aspirate and MRSᵃ

CI: confidence interval; FN: false negative; FP: false positive; LC-mNAAT: low-complexity manual nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
a Studies are sorted by increasing sensitivity.
Should LC-mNAATs on stool be used to diagnose pulmonary TB in children with signs and symptoms of pulmonary TB, against an MRS?
One study (144 participants, including seven with pulmonary TB) assessed the accuracy of LC-mNAATs for detecting pulmonary TB using stool against a MRS (Fig. 2.2.1.6). The sensitivity was 100% and specificity was 92%. The certainty of evidence was very low for sensitivity and moderate for specificity. Due to limited data, a recommendation on using LC-mNAATs with stool for detection of pulmonary TB was not made.
Fig. 2.2.1.6 Forest plot of LC-mNAAT sensitivity and specificity for detection of pulmonary TB in stool and MRS

CI: confidence interval; FN: false negative; FP: false positive; LC-mNAAT: low-complexity manual nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
Detection of TB meningitis
Should LC-mNAATs on CSF be used to diagnose TB meningitis in adults and adolescents with signs and symptoms of TB meningitis, against an MRS?
Two studies (70 participants, including three with TB meningitis) assessed the accuracy of LC-mNAATs for detecting TB meningitis using CSF and an MRS (Fig. 2.2.1.7). Estimated sensitivity and specificity were both 100% in one study, and 0% and 97%, respectively, in the other. The certainty of evidence was very low for sensitivity and low for specificity. Due to limited data, a recommendation on using LC-mNAATs with CSF for detection of TB meningitis was not made.
Fig. 2.2.1.7 Forest plot of LC-mNAAT sensitivity and specificity for detection of TB meningitis in CSF and MRS

CI: confidence interval; CSF: cerebrospinal fluid; FN: false negative; FP: false positive; LC-mNAAT: low-complexity manual nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
Detection of extrapulmonary TB
Should LC-mNAATs on lymph node tissue be used to diagnose lymph node TB in adults and adolescents with signs and symptoms of lymph node TB, against an MRS?
Three studies (95 participants, including 35 people with TB) assessed the accuracy of LC-mNAATs for detecting lymph node TB using lymph node tissue from biopsy and an MRS (Fig. 2.2.1.8). The estimated sensitivities were between 93% and 100%, and specificities were between 88% and 100%. The summary sensitivity was 94.3% (95% CI: 79.8–98.6), and the summary specificity was 90.0% (95% CI: 79.5–95.4). The certainty of evidence was low for both sensitivity and specificity. Due to limited data, a recommendation on using LC-mNAATs with lymph node tissue for the detection of lymph node TB was not made.
Fig. 2.2.1.8 Forest plot of LC-mNAAT sensitivity and specificity for detection of lymph node TB in lymph node tissue and MRS
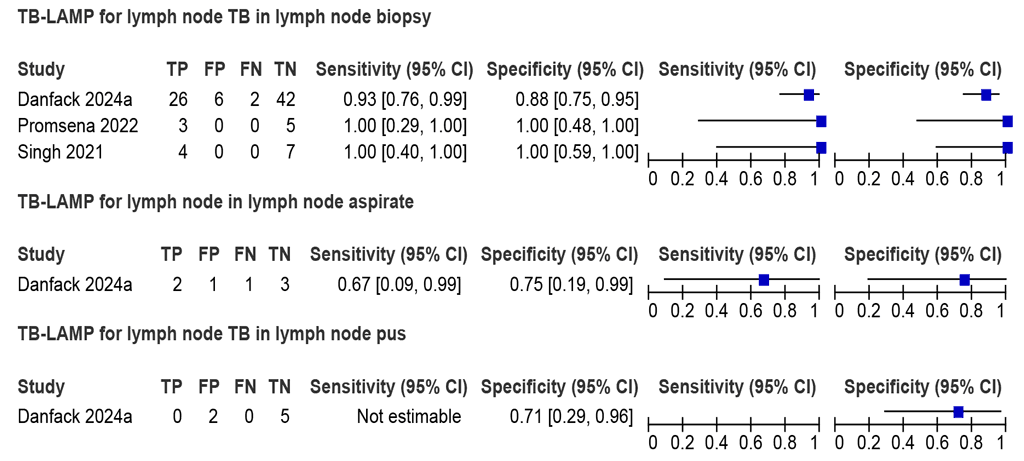
CI: confidence interval; FN; false negative; FP: false positive; LC-mNAAT: low-complexity manual nucleic acid amplification test; LN: lymph node; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
Should LC-mNAATs on pleural fluid be used to diagnose pleural TB in adults and adolescents with signs and symptoms of pleural TB, against an MRS?
Two studies (292 participants, including 37 people with TB) assessed the accuracy of LC-mNAATs for detecting pleural TB using pleural fluid and an MRS (Fig. 2.2.1.9). Estimated sensitivities were 48% and 75%, and estimated specificities were 89% and 96%. Due to limited data, a recommendation on using LC-mNAATs with pleural fluid for detection of pleural TB was not made.
Fig. 2.2.1.9 Forest plot of LC-mNAAT sensitivity and specificity for detection of pleural TB in pleural fluid and MRS
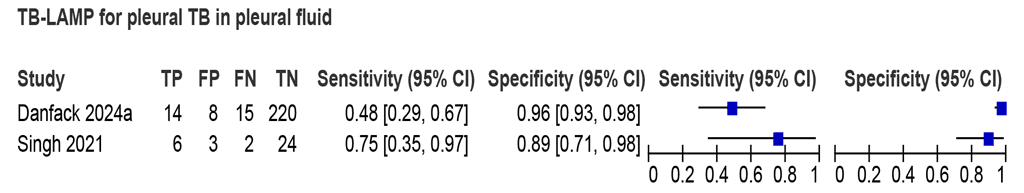
CI: confidence interval; FN: false negative; FP: false positive; LC-mNAAT: low-complexity manual nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
Should LC-mNAATs on synovial fluid be used to diagnose bone or joint TB in adults and adolescents with signs and symptoms of bone or joint TB, against an MRS?
One study (five participants, including one case) assessed the accuracy of LC-mNAATs for detecting bone or joint TB using synovial fluid and an MRS (Fig. 2.2.1.10). Estimated sensitivity and specificity were both 100%. Due to limited data, a recommendation on using LC-mNAATs with synovial fluid for detection of bone or joint TB was not made.
Fig. 2.2.1.10 Forest plot of LC-mNAAT sensitivity and specificity for detection of bone or joint TB in synovial fluid and MRS

CI: confidence interval; FN: false negative; FP: false positive; LC-mNAAT: low-complexity manual nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
Should LC-mNAATs on urine be used to diagnose genitourinary TB in adults and adolescents with signs and symptoms of genitourinary TB, against an MRS?
One study (32 participants, including two people with TB) assessed the accuracy of LC-mNAATs for detecting genitourinary TB using urine and an MRS (Fig. 2.2.1.11). Estimated sensitivity and specificity were 50% and 100%, respectively. Due to limited data, a recommendation on using LC-mNAATs with urine for detection of genitourinary TB was not made.
Fig. 2.2.1.11 Forest plot of LC-mNAAT sensitivity and specificity for detection of genitourinary TB in urine and MRS

CI: confidence interval; FN: false negative; FP: false positive; LC-mNAAT: low-complexity manual nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
Cost–effectiveness analysis
This section deals with the following additional question:
What are the comparative costs, affordability and cost–effectiveness of implementation of LC-mNAATs?
A systematic review commissioned by WHO aimed to identify, evaluate and summarize the findings of available economic evidence on LC-mNAATs, among other technologies. The systematic review provided an in-depth analysis of the financial implications and cost– effectiveness of implementing TB LAMP in diverse settings. Through a range of economic analyses, including cost–utility, cost–benefit and cost–affordability assessments, this study contributes valuable insights into the potential role of TB LAMP in TB diagnostics.
After removing 638 duplicate studies from those identified in the original search, 1990 unique studies remained. Of these, six studies were included in the final systematic review. Studies that did not involve people with TB, used TB LAMP as a diagnostic intervention or did not contain cost data were excluded. Of the six included studies, one performed a cost–utility analysis, and two performed a cost–affordability analysis. The three other studies estimated the cost of TB LAMP.
All included studies were conducted in LMIC. Specifically, two studies were conducted in Thailand, one in Malawi, one in both Malawi and Viet Nam, and one each in India and Cameroon. The studies were conducted between 2014 and 2021 across various settings, such as outpatient departments at health centres, peripheral laboratories, a laboratory for the development of modified TB LAMP, and prisons and villages involving inmates and refugees. One study used sputum samples from people known to have TB, and another used fine needle aspiration of lymph node samples from HIV-positive patients with TB lymphadenitis. The other four studies used sputum samples from people with presumptive TB.
According to the three costing studies, the cost per test ranged from US$ 1 to US$ 19 (all values in 2024 US dollars). All these studies used in-house techniques and were not using the commercially available TB LAMP test. The reviewed studies found that factors such as batching scenarios and larger test capacity influence the per-test cost, with the cost per test decreasing in specific scenarios. Testing volumes, location and operational parameters can also affect the cost. Notably, the cost–utility analysis positioned TB LAMP favourably in terms of cost–effectiveness compared with other diagnostic algorithms.
The findings of the cost–utility analysis suggested that TB LAMP, followed by DST, is not only effective but also cost-saving when compared with the standard diagnostic approach (i.e. smear, culture and DST). These results provide valuable insights for health care practitioners and policymakers in terms of optimizing TB diagnostic strategies while considering cost–effectiveness.
One cost–affordability analysis, conducted in peripheral laboratories in Malawi and Viet Nam, highlighted the economic considerations of implementing TB LAMP and Xpert MTB/ RIF. The study showed that per-test costs for TB LAMP were lower than those for Xpert MTB/ RIF. However, the potential financial burden of widespread implementation underscored the importance of cost–effectiveness assessments in shaping diagnostic strategies. For more details see Web Annex B.9.
The reviewed studies had some limitations, such as variations in settings, sample sources and comparators, which may influence the generalizability of findings. Additionally, the cost– affordability analysis underscores the financial implications of nationwide implementation, suggesting the need for careful budgetary planning and allocation. Furthermore, the Global Drug Facility’s recent decrease in the price of TB LAMP (new price, US$ 6) may have an impact on the results of economic evaluations, potentially enhancing the cost–effectiveness and affordability of implementing TB LAMP in diverse settings.
These collective findings suggest that TB LAMP holds promise as a cost-effective and efficient diagnostic tool for TB when integrated into broader diagnostic algorithms, particularly in resource-constrained settings.
More details on the economic evaluation of LC-mNAATs are available in Web Annex B.9.
User perspective
This section deals with the following question:
Are there implications for user preferences and values, acceptability, feasibility, patient equity and human rights from the implementation of LC-mNAATs?
The findings from the studies that focused on LC-aNAATs are largely applicable to LC-mNAATs, with the caveat of slightly lower sensitivity and the lack of ability to detect resistance to rifampicin.
A systematic review of the qualitative evidence of LC-NAATs (Web Annex B.10) did not identify any studies focused on LC-mNAATs (acknowledging that a few studies did not specify the type of NAAT they were focusing on).
However, selected findings from the interview study, did focus specifically on TB-LAMP:
- In 2018, Nigeria adopted the use of TB LAMP (along with GeneXpert and Truenat). At the time of the interview, there were 199 TB LAMP machines in Nigeria. These are placed both in sites where GeneXpert is available, to decrease workload, and in peripheral laboratories where the infrastructure is insufficient to accommodate GeneXpert. Positive results from TB LAMP are sent to the nearest site with a GeneXpert or Truenat machine for DST.
- The Philippines National TB Programme (NTP) guidelines advise the use of TB LAMP as an alternative primary diagnostic test in settings where access to GeneXpert is limited and that currently rely on sputum transport riders (STRiders). TB LAMP was piloted in 2019 (April to September) and 2020 (October to February 2021) in a rural health unit, polyclinic and private hospital, and for TB mass screening in a rural health unit. The pilot implementation only tested sputum with TB LAMP and did not test for TB in MDR risk groups, children or people living with HIV. According to a laboratory manager, there are about six or seven TB LAMP machines in the Philippines. These are not currently in use but could be if there was support for buying reagents.
According to Nigeria’s TB LAMP guidelines, the following criteria should be used to prioritize sites for TB LAMP testing:
- facilities with high workload;
- facilities with or without an existing molecular platform;
- laboratories with adequate space and infrastructure;
- availability of qualified medical laboratory personnel;
- an adequate number of medical laboratory personnel; and
- a storage facility (e.g. refrigerator) for laboratory and administrative supply.
User preferences and values
An interview study on TB LAMP involving sites in Nigeria and the Philippines found the following views of laboratory personnel and programme officers:
- TB LAMP is making laboratory work easier over time through familiarity and because it clears the workbench;
- compared to SSM, TB LAMP is easier to use; and
- in direct comparison with Xpert Ultra, TB LAMP is more hands-on and requires more user steps and time for preparing and processing specimens.
Acceptability
Acceptability of the test seems to be slightly reduced because TB LAMP cannot test for rifampicin resistance and has no multiplexing opportunities.
Feasibility
Summarized findings from the interview study on TB LAMP are as follows. TB LAMP improves access to TB diagnosis for people who would otherwise have been missed, because it can run in laboratories with limited infrastructure, has high throughput, is more accurate than SSM and reduces workload at GeneXpert sites. It allows decentralization of testing and therefore has the potential to reduce catastrophic cost to patients. However, TB LAMP adoption decisions are also driven by donors and investment considerations.
Overall, TB LAMP allows staff to carry out more tests, and faster. Its high throughput contributes to acceptability and utilization. Laboratory staff in Nigeria are given incentives for the number of tests they carry out, making use of TB LAMP even more attractive. These incentives also support swift action when maintenance or repair of the devices is needed.
Programmatic feasibility seems to be less of a concern than with GeneXpert. Compared with implementing GeneXpert, programme officers find TB LAMP more feasible to implement due to its lower requirements for infrastructure, skills level and maintenance. As with all LC-NAATs, staffing and reagent supply issues challenge its use. TB LAMP’s impact on the overall turnaround time for DR-TB diagnosis, MDR-TB treatment initiation and loss to follow-up at sites without Xpert Ultra testing depends on the efficiency and robustness of the sample transport or referral system.
More details on the qualitative evaluation of LC-mNAATs are available in Web Annex B.10.
Implementation considerations
- Diagnostic products in the low-complexity classes of tests should be prequalified by WHO or approved by another regulator before clinical use.
- Diagnostic test manufacturers, laboratory and programme managers, and policy-makers should be educated on the WHO PQ process for TB IVDs.
- Ensuring sufficient volume and specimen quality is important to obtain accurate results.
- Safe waste disposal of used test consumables needs to be planned in advance to minimize environmental risk.
Monitoring and evaluation
- Track errors and invalid test result rates for currently recommended products and new products to be introduced in this class.
- Monitor the proportion of people with bacteriologically confirmed TB without rifampicinresistance reflex testing or access to further DST over time.
Research priorities
- Evaluate the performance of this class using alternative sample types for paediatric TB (e.g. gastric and nasopharyngeal aspirates, stool, induced sputum, BAL) and extrapulmonary TB.
- Evaluate the impact of LC-mNAAT testing on patient-important outcomes (cure, mortality, time to diagnosis and time to start of treatment).
- Evaluate the effect of sample concentration approaches (e.g. centrifugation) and volume on the performance of LC-mNAAT technologies, including in extrapulmonary TB sample types.
- Evaluate the impact on incremental accuracy and case detection of alternative sample types that are easier to collect.
- Develop a test in this class that can detect TB drug resistance.
- Review the field performance of the current technologies used in programmatic settings.
- Conduct operational research to ensure that tests are used optimally in intended settings.
- Evaluate the different classes of tests, including LC-mNAATs, to determine which classes or testing strategies yield superior diagnostic accuracy, cost–effectiveness and impact on equity and acceptability.
- Identify an improved reference standard that accurately defines TB disease in children, paucibacillary specimens and people who cannot produce sputum, because the sensitivity of all available diagnostics is suboptimal.
- Assess the budget impact and cost–effectiveness of LC-mNAATs compared with other classes of tests.
- Develop and apply standardized methods for assessment of costs and cost–effectiveness, to improve comparability and scope of economic evidence.

 Feedback
Feedback