Book traversal links for WHO TB KNOWLEDGE SHARING PLATFORM
2.1.1 LC-aNAATs for detection of TB and resistance to rifampicinNEW
Rapid detection of TB and rifampicin resistance is a critical global priority. Over a decade ago, the first recommendation on molecular testing for the diagnosis of TB and detection of resistance significantly transformed the TB diagnostic landscape. These technologies have proven highly accurate compared with smear microscopy, and they can detect rifampicin resistance rapidly. They do not require highly skilled individuals or designated molecular laboratory infrastructure for testing. In addition, they are largely automated after sample loading, up to the final report generation. These features make this class of low-complexity automated tests appealing for use in low- and middle-income countries (LMIC).
Uptake of these technologies has been slowed by barriers related to costs, the supply chain, equipment maintenance and technical support. The lack of a healthy competitive environment has also been a contributory factor. The WHO Prequalification (PQ) programme for TB in vitro diagnostics (IVDs) has opened a pathway to allow more products to come to market and ensure quality. The current guidelines facilitated this process with the introduction of classbased recommendations for low-complexity NAATs. WHO PQ assessment progress for all low-complexity NAATs is reported on the WHO PQ website.⁴
Diagnostic class description
The features shown in Table 2.1.1.1 define the class of LC-aNAATs.:
Table 2.1.1.1 Class criteria for LC-aNAATs
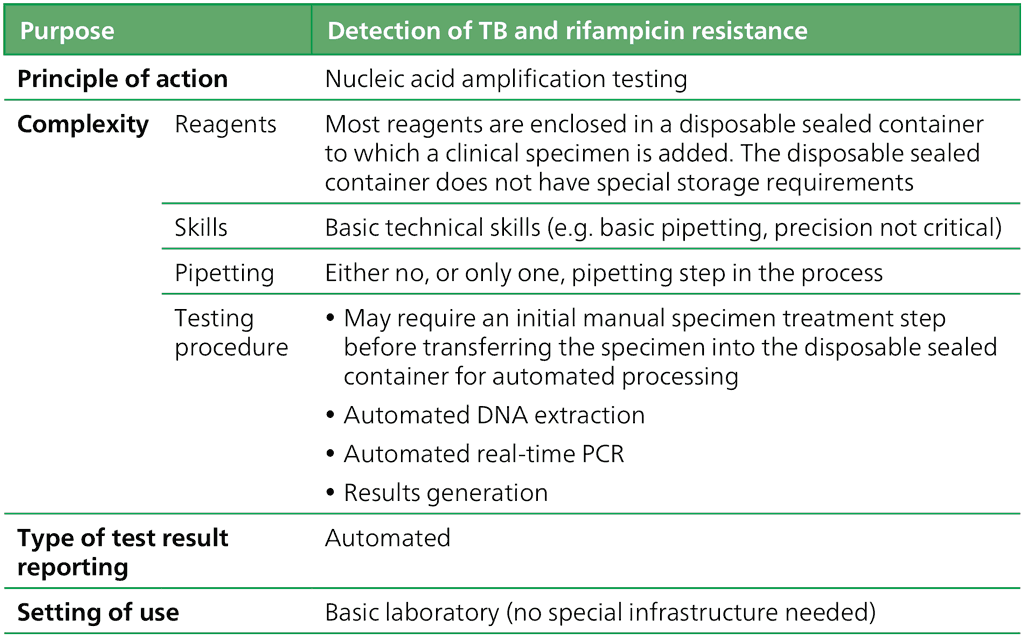
DNA: deoxyribonucleic acid; LC-aNAAT: low-complexity automated nucleic acid amplification test; PCR: polymerase chain reaction; TB: tuberculosis.
The products for which eligible data met the class-based performance criteria for LC-aNAATs were:
- Xpert MTB/RIF Ultra (Cepheid, Sunnyvale, United States of America [USA]) – for pulmonary TB, extrapulmonary TB and resistance to rifampicin; and
- Truenat MTB Plus and Truenat MTB-RIF Dx (Molbio, Goa, India) – for pulmonary TB and resistance to rifampicin.
Data on Truenat MTB Plus and MTB-RIF Dx were more limited than those for Xpert Ultra.
Regulatory approval from national regulatory authorities or other relevant bodies is required before implementation of these diagnostic tests. Extrapolation to other brand-specific tests cannot be made, and any new in-class technologies, or new indications for technologies currently included in the class, will need to be evaluated by WHO PQ and WHO/GTB, respectively.
The publication WHO operational handbook on tuberculosis. Module 3: Diagnosis describes the tests included in this class.
Recommendations

Remarks
- For adults, respiratory samples include sputum (expectorated or induced), tracheal aspirate or bronchoalveolar lavage (BAL).
- The term “person screened positive” refers to a person in whom a screening test has yielded a positive result.⁵
- Children and specifically children living with HIV are discussed in the section on the concurrent use of initial TB diagnostic tests in children.
- Adults and adolescents living with HIV are discussed in the section on the concurrent use of initial TB diagnostic tests in people living with HIV.
- The products for which eligible data met the class-based performance criteria for LC-aNAATs for this recommendation were Xpert MTB/RIF Ultra (Cepheid, Sunnyvale, United States of America [USA]) and Truenat MTB Plus (Molbio, Goa, India). Data on Truenat MTB Plus and MTB-RIF Dx were more limited than those for Xpert Ultra.

Remarks
- This recommendation applies to all people living with HIV.
- The recommendation was extrapolated to children based on the generalization of data from adults and limited data from children. For children, respiratory samples include sputum, BAL, induced sputum, nasopharyngeal aspirate and gastric aspirate.
- The recommendation was extrapolated to people with extrapulmonary TB based on the generalization of data from adults with pulmonary TB.
- The products for which eligible data met the class-based performance criteria for LC-aNAATs for this recommendation were Xpert MTB/RIF Ultra (Cepheid, Sunnyvale, United States of America [USA]) and Truenat MTB-RIF Dx (Molbio, Goa, India). Data on MTB-RIF Dx were more limited than those for Xpert Ultra.

Remarks
- This recommendation applies to all people with signs and symptoms of TB meningitis, including people living with HIV and children.
- Where possible, culture may be performed in addition to automated NAAT testing, to maximize the opportunity for diagnosis and detection of DR-TB.
- The product for which eligible data met the class-based performance criteria for LC-aNAATs for this recommendation was Xpert MTB/RIF Ultra (Cepheid, Sunnyvale, United States of America [USA]). Data on Truenat MTB Plus and MTB-RIF Dx were limited and variable and thus were insufficient for evaluation.
Remarks
- This recommendation applies to all people with signs and symptoms of the respective form of extrapulmonary TB, including people living with HIV and children.
- Data on the performance of LC-aNAATs when used with urine and blood samples were limited or inconsistent.
- Where possible, culture may be performed in addition to automated NAAT testing, to maximize the opportunity for diagnosis and detection of DR-TB.
- The product for which eligible data met the class-based performance criteria for LC-aNAATs for this recommendation was Xpert MTB/RIF Ultra (Cepheid, Sunnyvale, United States of America [USA]). Data on Truenat MTB Plus and MTB-RIF Dx were limited and variable and thus were insufficient for evaluation.
Justification and evidence
WHO/GTB initiated an update of the previous guidelines and commissioned a systematic review on the use of LC-aNAATs (Xpert Ultra, Truenat MTB Plus and Truenat MTB-RIF Dx assays) for the diagnosis of TB and resistance to rifampicin in people with signs and symptoms of TB, or who screened positive for TB. The data on the performance of LC-aNAATs alone in these populations, compared with smear microscopy and culture, are presented in Web Annexes B.1–B.4. Recommendations on concurrent testing for children and people living with HIV supersede the use of LC-aNAATs alone in these populations (see Section 2.3 of this document).
Detection of pulmonary TB
Should LC-aNAATs on respiratory samples be used to diagnose pulmonary TB in adults and adolescents with signs and symptoms or who screened positive for pulmonary TB, against a microbiological reference standard?
Thirty-five studies (14 845 participants) assessed diagnostic accuracy using sputum specimens and comparing with a microbiological reference standard (MRS); however, one of those studies had no people with TB (Zar 2019) and so sensitivity was not estimable. The sensitivities in the remaining 34 studies (14 840 participants) included in the meta-analysis were between 54% and 100%, and the specificities were between 71% and 100% (Fig. 2.1.1). The summary sensitivity was 90.4% (95% confidence interval [CI]: 88.0–92.4), and the summary specificity was 94.9% (95% CI: 93.0–96.3). The certainty of evidence for sensitivity and specificity was graded as “high”. For more details, see Web Annex B.1.
Fig. 2.1.1. Forest plot of LC-aNAAT sensitivity and specificity for detection of pulmonary TB in sputum samples and MRSᵃ
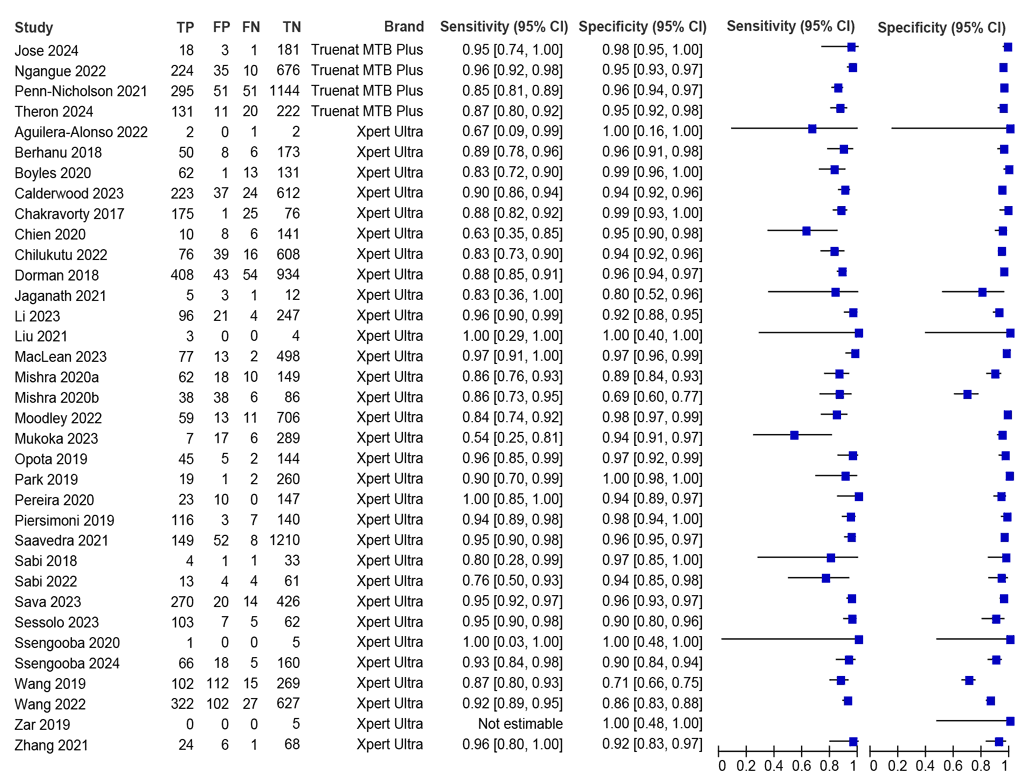
CI: confidence interval; FN: false negative; FP: false positive; LC-aNAAT: low-complexity automated nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
a Studies are sorted by assay and author.
Detection of rifampicin resistance
Should LC-aNAATs on respiratory samples be used to diagnose rifampicin resistance in adults and adolescents with signs and symptoms or who screened positive for pulmonary TB, against an MRS?
Of the 13 studies (2553 participants) that evaluated sputum specimens, sensitivity for detecting rifampicin resistance was not estimable for two studies (Fig. 2.1.2). The sensitivities in the remaining 11 studies (2540 participants) included in the meta-analysis were between 53% and 100%, and the specificities were between 97% and 100%. The summary sensitivity was 95.1% (95% CI: 83.1–98.7), and the summary specificity was 98.1% (95% CI: 97.0–98.7). Only two of the 11 included studies assessed Truenat MTB-RIF Dx; one of them, a study from a single country, had a sensitivity outside of confidence interval limits (53%). Nevertheless, overall, the certainty of evidence for both sensitivity and specificity was considered high.
Fig. 2.1.2. Forest plot of LC-aNAAT sensitivity and specificity for detection of rifampicin resistance in respiratory specimens and MRSᵃ
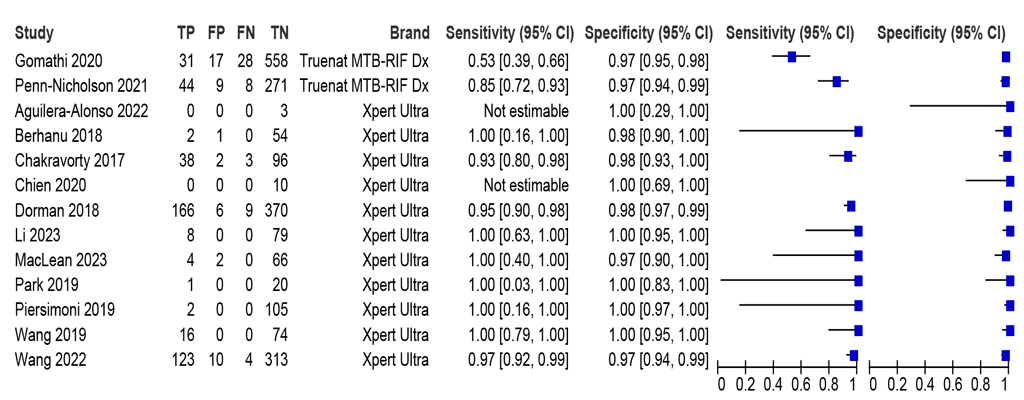
CI: confidence interval; FN: false negative; FP: false positive; LC-aNAAT: low-complexity automated nucleic acid amplification test; MRS: microbiological reference standard; TN: true negative; TP: true positive.
a Studies are sorted by assay and author.
Detection of TB meningitis
Should LC-aNAATs on cerebrospinal fluid (CSF) be used to diagnose TB meningitis in adults with signs and symptoms of TB meningitis, against an MRS?
LC-aNAAT summary sensitivity and specificity were 88.2% (95% CI: 83.7–91.6) and 96.0% (95% CI: 86.8–98.9), respectively, based on 16 Xpert Ultra studies (1684 participants); the certainty of evidence was high for sensitivity and moderate for specificity (Fig. 2.1.3). Only data on Xpert Ultra were included in the evaluation to answer this population, intervention, comparator and outcome (PICO) question. Of note, trace results from Xpert Ultra were considered positive and formed a significant proportion of positive results (16–63%). Data on Truenat were limited and variable and thus were not included. For more details, see Web Annex B.3.
Fig. 2.1.3. Forest plot of LC-aNAAT sensitivity and specificity for detection of TB meningitis in cerebrospinal fluid and MRSᵃ
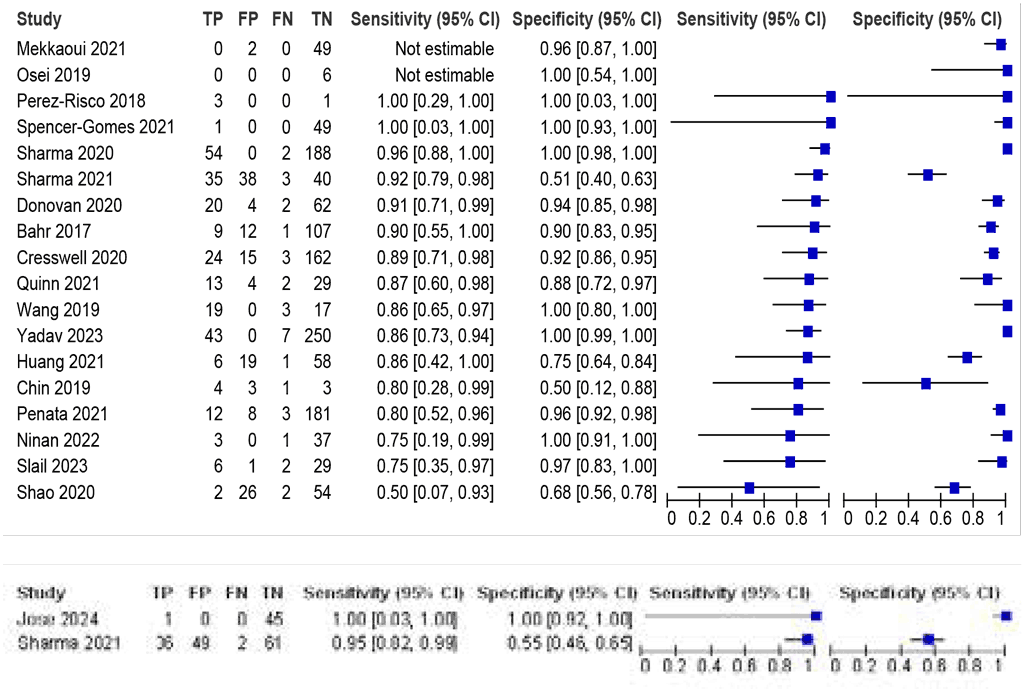
CI: confidence interval; CSF: cerebrospinal fluid; FN: false negative; FP: false positive; LC-aNAAT: low-complexity automated nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
a Studies are sorted by decreasing sensitivity.
Detection of extrapulmonary TB
Should LC-aNAATs on lymph node fluid be used to diagnose lymph node TB in adults and adolescents with signs and symptoms of lymph node TB, against an MRS?
LC-aNAAT summary sensitivity and specificity from nine Xpert Ultra studies (445 participants) to diagnose lymph node TB in lymph node fluid in adults and adolescents with signs and symptoms of lymph node TB (Fig. 2.1.4) were 85.3% (95% CI: 73.4–92.4) and 74.1% (95% CI: 63.5–82.5), respectively. The certainty of evidence was low for sensitivity and very low for specificity. Only data on Xpert Ultra were included in the evaluation to answer this PICO question. The diagnostic accuracy of LC-aNAATs against a composite reference standard (CRS) that comprised the MRS plus patients who received clinical diagnoses (but were bacteriologically unconfirmed) was also considered. The use of the CRS markedly increased specificity to 97.4% (95% CI: 82.2–99.7) but decreased sensitivity to 71.3% (95% CI: 64.3–77.4), highlighting the known challenges with culture-based confirmation of TB with this sample type (see Web Annex B.3). Data on Truenat were limited and thus were not included.
Fig. 2.1.4. LC-aNAAT sensitivity and specificity for detection of lymph node TB in lymph node aspirate and MRSᵃ
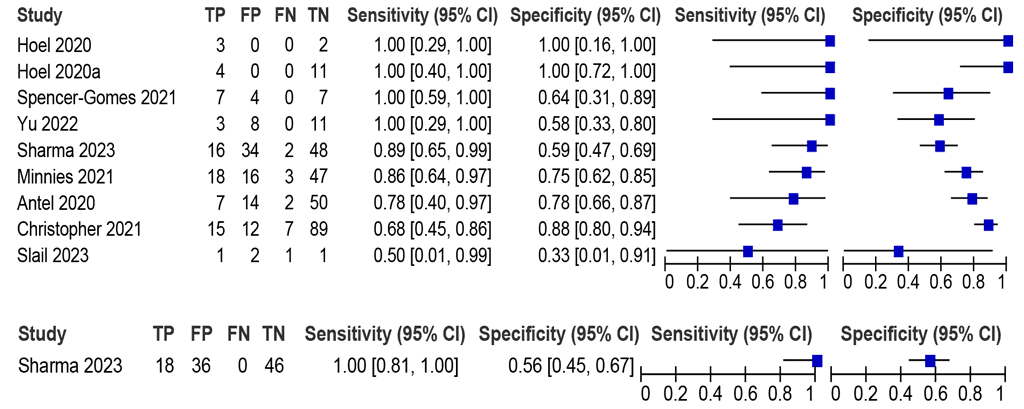
CI: confidence interval; FN: false negative; FP: false positive; LC-aNAAT: low-complexity automated nucleic acid amplification test; LN: lymph node; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
a Studies are sorted by decreasing sensitivity.
Should LC-aNAATs on pleural tissue be used to diagnose pleural TB in adults and adolescents with signs and symptoms of pleural TB, against an MRS?
From two Xpert Ultra studies (105 participants), LC-aNAAT sensitivities were 80% and 100%, and specificities were 75% and 86% (Fig. 2.1.5); the certainty of evidence was low for sensitivity and very low for specificity. Only data on Xpert Ultra were included in the evaluation to answer this PICO question, as data on Truenat were not available. Given known challenges with culture-based confirmation of TB using this sample, the data using the CRS were also considered. The use of the CRS increased specificity of the LC-aNAAT on pleural tissue to 94–97%, but it decreased sensitivity to 54–81%⁶ (see Web Annex B.3).
Fig. 2.1.5. LC-aNAAT sensitivity and specificity for detection of pleural TB in pleural tissue and MRSᵃ

CI: confidence interval; FN: false negative; FP: false positive; LC-aNAAT: low-complexity automated nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
a Studies are sorted by decreasing sensitivity.
Should LC-aNAATs on pleural fluid be used to diagnose pleural TB in adults and adolescents with signs and symptoms of pleural TB, against an MRS?
LC-aNAAT summary sensitivity and specificity were 74.0% (95% CI: 60.8–83.9) and 88.1% (95% CI: 78.8–93.6), respectively, from 13 Xpert Ultra studies (1041 participants) (Fig. 2.1.6). The certainty of evidence was low for sensitivity and very low for specificity. Only one study (Jose 2024) provided accuracy estimates for pleural fluid for Truenat MTB Plus (88 participants), with sensitivity of 100% (95% CI: 0.03–100) and specificity of 100% (95% CI: 0.95–100). Similar to lymph node fluid and pleural tissue, the data using the CRS were also considered for this sample type. The use of the CRS increased specificity of LC-aNAATs on pleural fluid to 99.2% (95% CI: 95.2%–99.9%) but decreased sensitivity to 71.3% (95% CI: 64.3%–77.4%) (see Web Annex B.3). Only data on Xpert Ultra were included in the evaluation to answer this PICO question. Data on Truenat were limited.
Fig. 2.1.6. LC-aNAAT sensitivity and specificity for detection of pleural TB in pleural fluid and MRSᵃ
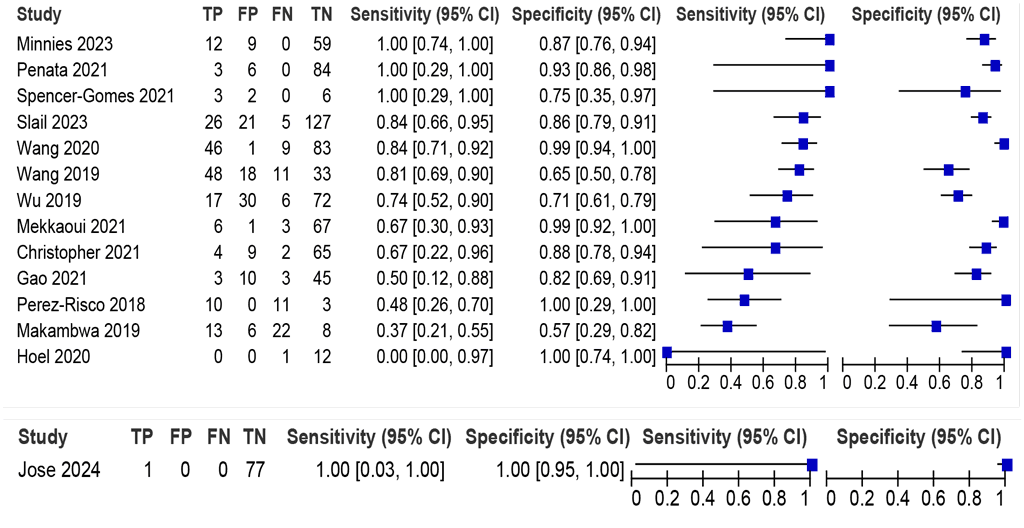
CI: confidence interval; FN: false negative; FP: false positive; LC-aNAAT: low-complexity automated nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
a Studies are sorted by decreasing sensitivity.
Should LC-aNAATs on synovial fluid be used to diagnose bone or joint TB in adults and adolescents with signs and symptoms of bone or joint TB, against an MRS?
LC-aNAAT summary sensitivity and specificity were 96.6% (95% CI: 87.2–99.1) and 91.1% (95% CI: 80.8–96.2), respectively, from three Xpert Ultra studies (126 participants) (Fig. 2.1.7); the certainty of evidence was low. Similar to other extrapulmonary TB sample types, the data using the CRS were also considered. The use of the CRS increased specificity of the LC-aNAAT on synovial fluid to 97.0% (95% CI: 85.0–100.0), whereas the impact on sensitivity was minimal (96%) and largely involved tightening of the confidence interval (95% CI: 91–99%). Only data on Xpert Ultra were included in the evaluation to answer this PICO question. Data on Truenat were limited.
Fig. 2.1.7. LC-aNAAT sensitivity and specificity for detection of bone or joint TB in synovial fluid or tissue and MRSᵃ
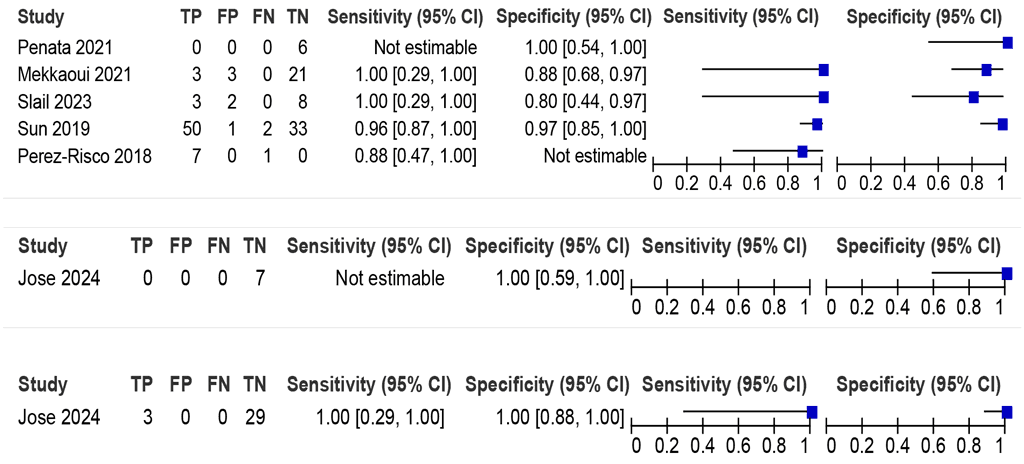
CI: confidence interval; FN: false negative; FP: false positive; LC-aNAAT: low-complexity automated nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
a Studies are sorted by decreasing sensitivity.
Should LC-aNAATs on peritoneal fluid be used to diagnose peritoneal TB in adults and adolescents with signs and symptoms of peritoneal TB, against an MRS?
The sensitivities of the LC-aNAATs ranged from 33% to 67%, and the specificities from 94% to 100%, from three Xpert Ultra studies (69 participants); the certainty of evidence was very low for sensitivity and low for specificity (Fig. 2.1.8). Only data on Xpert Ultra were included in the evaluation to answer this PICO question. Data on Truenat were limited.
Fig. 2.1.8. LC-aNAAT sensitivity and specificity for detection of peritoneal TB in peritoneal fluid and MRSᵃ
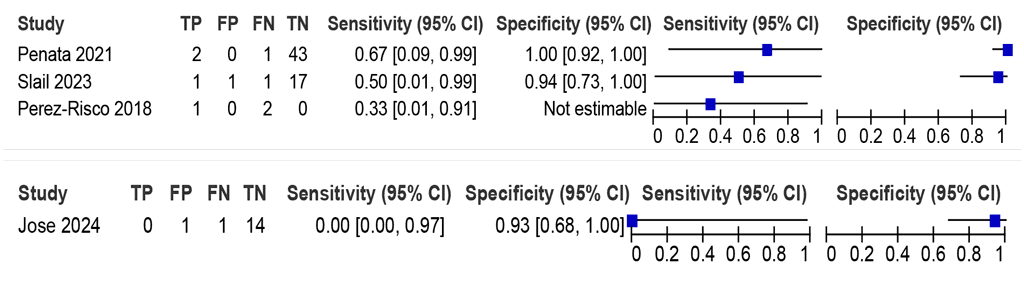
CI: confidence interval; FN: false negative; FP: false positive; LC-aNAAT: low-complexity automated nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
a Studies are sorted by decreasing sensitivity.
Should LC-aNAATs on pericardial fluid be used to diagnose pericardial TB in adults and adolescents with signs and symptoms of pericardial TB, against an MRS?
LC-aNAAT summary sensitivity and specificity were 84.0% (95% CI: 73.9–90.7) and 86.6% (95% CI: 79.5–91.5), respectively, from three Xpert Ultra studies (202 participants); certainty of evidence was low for both sensitivity and specificity (Fig. 2.1.9).
Fig. 2.1.9. LC-aNAAT sensitivity and specificity for detection of pericardial TB in pericardial fluid and MRSᵃ
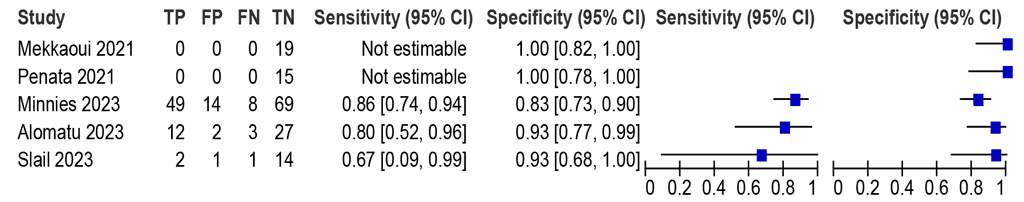
CI: confidence interval; FN: false negative; FP: false positive; LC-aNAAT: low-complexity automated nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
a Studies are sorted by decreasing sensitivity.
Should LC-aNAAT on extrapulmonary specimens be used to diagnose rifampicin resistance in adults and adolescents with presumed extrapulmonary TB?
LC-aNAAT summary sensitivity and specificity were 100.0% (95% CI: 93.4–100.0) and 99.4% (95% CI: 92.1–100.0), respectively, from 13 Xpert Ultra studies (446 participants) (Fig. 2.1.10); certainty of evidence was high for both sensitivity and specificity.
Fig. 2.1.10. LC-aNAAT sensitivity and specificity for detection of pericardial TB in pericardial fluid and MRSᵃ
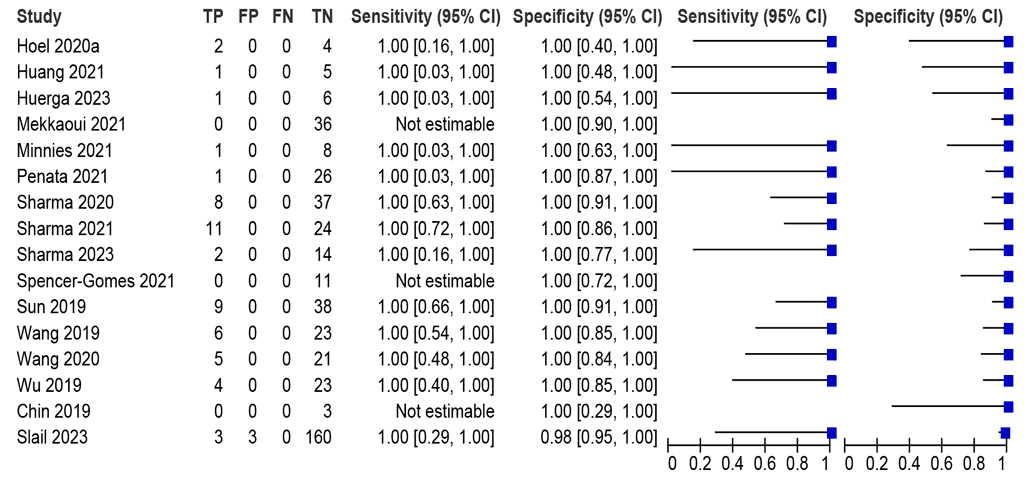
CI: confidence interval; FN: false negative; FP: false positive; LC-aNAAT: low-complexity automated nucleic acid amplification test; MRS: microbiological reference standard; TB: tuberculosis; TN: true negative; TP: true positive.
a Studies are sorted by sensitivity.
Cost–effectiveness analysis
This section deals with the following additional question:
What are the comparative costs, affordability and cost–effectiveness of implementation of LC-aNAATs?
WHO commissioned a systematic review to identify, evaluate and summarise the evidence on cost, affordability and cost-effectiveness of LC-aNAATs, among other technologies.
A total of 1534 studies were identified in the original search; after removing duplicates, 736 potentially relevant studies were screened. Of these, 107 were assigned for full-text review and were evaluated against the inclusion and exclusion criteria, and 29 studies were included in the final systematic review. Of the 29 included studies, 22 (76%) assessed Xpert MTB/RIF, six (21%) assessed Xpert Ultra, and one study (3%) evaluated Truenat tests (both Truenat MTB Plus and Truenat MTB-RIF Dx). Ten of the studies evaluating Xpert MTB/RIF were cost– effectiveness analyses, and 12 were cost analyses. All the included Xpert Ultra studies and the study evaluating Truenat were cost–effectiveness analyses.
The cost-effectiveness analysis on Xpert was considered because of similarity between two technologies and scarcity of the data on Ultra.
The studies included in the review were diverse, were conducted across various settings and covered all income levels. This broad spectrum of research provided a comprehensive view of the economic evidence on LC-aNAATs, with a focus on adults. Only three cost–effectiveness analyses included children. There were various comparator tests, including smear and culture. Most of the studies included sputum specimens. Six of the 10 cost–effectiveness analyses on Xpert MTB/RIF presented results in natural units (additional people with TB detected), and the other four presented utility outcomes (quality-adjusted life years and disability-adjusted life years [DALYs]).
The findings from the cost–effectiveness analyses showed that Xpert MTB/RIF was generally cost effective across the included studies when compared with smear or culture, except for one study from Thailand, where TB LAMP was the dominant strategy. In contrast, there was more heterogeneity in the methodology used in the cost–effectiveness studies for Xpert Ultra, and the findings showed that, for the Xpert Ultra versus sputum smear microscopy (SSM), an incremental cost–effectiveness ratio (ICER) ranged from US$ 72.72 to US$ 160.23 per DALY averted. In the only study on Truenat, it was found to be cost effective for children in India compared with Xpert MTB/RIF, with an ICER of US$ 94.72 per DALY averted.
In general LC-aNAATs are likely to be cost effective across various settings when compared with SSM and culture.
More details on the economic evaluation of LC-aNAATs are available in Web Annex B.9.
User perspective
This section deals with the following question:
Are there implications for user preferences and values, patient equity, accessibility, feasibility and human rights from the implementation of Xpert MTB/ RIF and Xpert Ultra?
This review included 49 qualitative studies, of which 17 were identified in the updated search (since 2022). All studies about LC-aNAATs for detection of TB and DR-TB were conducted in high TB burden settings in Africa, Asia and Eastern Europe. Two studies provided user perspectives on Xpert Ultra and the rest on Xpert MTB/RIF or other rapid molecular tests. The studies about Xpert Ultra were conducted in Africa and Eastern Europe and focused on all people with presumptive TB, DR-TB and extrapulmonary TB.
Although standard Xpert MTB/RIF has been superseded by Xpert Ultra and other rapid NAATs, qualitative evidence for the latter NAATs is limited. Whereas LC-NAATs are generally valued for their accuracy, ease of use and potential to reduce time to diagnosis, the most recent generation of NAATs, such as Xpert Ultra, are valued for their greater accuracy in hard-to-diagnose patients, ease of implementation on existing GeneXpert platforms and ease of integration with rapid testing for other diseases. Challenges limiting the realization of these values for more recent NAATs are similar to those with Xpert MTB/RIF – that is, weak infrastructure, fragmented systems, heavy workloads, and limited availability of NAATs and their supplies. We recommend that qualitative studies be conducted to ascertain perspectives on concurrent use of NAATs.
There was high confidence in the evidence contributing to the findings of this review. More details on the qualitative evaluation of LC-aNAATs are available in Web Annex B.10.
User preferences and values
Findings from Xpert MTB/RIF studies showed that providers valued its utility in making a diagnosis of drug resistance in people living with HIV, accuracy and resulting confidence in the test, rapid turnaround times, low costs of diagnostic testing for patients, and improved patient– provider relationships. Providers also valued the diversity of sample types that can be analysed by the test. Laboratory personnel valued its ease of use, and they reported increased staff satisfaction compared with sputum microscopy. People with TB valued receiving an accurate diagnosis, avoiding diagnostic delays and having low costs associated with diagnostic testing. Compared with Xpert MTB/RIF, providers valued Xpert Ultra’s capacity for improving TB case detection among hard-to-diagnose patients (those with extrapulmonary TB, paediatric TB or coinfection with HIV) and detecting more people with TB.
Compared with Xpert MTB/RIF, providers valued Xpert Ultra for its ease of implementation and integration with testing for other diseases (made possible by its having been built on existing Xpert platforms). Acceptability of Xpert Ultra among providers seemed high, but there was uncertainty about its accuracy, potentially leading to reduced trust and litigation in the event of a false diagnosis.
Patient equity
The limited availability of Xpert Ultra in health facilities and the high costs incurred by patients and health facilities for its use were reported as concerns in terms of equity.
Acceptability
There were challenges to using Xpert MTB/RIF in the health care system. These challenges included underuse of the test and delays in the diagnostic pathway because of poor sample quality, insufficient resources and maintenance of the testing platforms, lack of functional data connectivity systems or record systems, inefficient patient flows, unavailability of updated clinical guidelines, and poor ownership of and accountability for the tests by health facilities. Overreliance on test results, rather than clinical judgement, and a lack of data-driven implementation processes were reported.
Access to the test may be limited owing to lack of sustainable funding, restrictions by donors, poor referral systems, dependence on outreach workers, unavailability of community TB diagnostic facilities and too many eligibility restrictions.
Feasibility
As with Xpert MTB/RIF, implementation of Xpert Ultra could be hindered by infrastructural problems, such as power outages, staff shortages, limited availability of transportation for sputum samples and limited availability of Xpert testing platforms in health facilities.
Implementation considerations
- Diagnostic products in the low-complexity classes of tests should be prequalified by WHO or approved by another regulator before clinical use.
- Diagnostic test manufacturers, laboratory and programme managers, and policy-makers should be educated on the WHO PQ process for TB IVDs (https://extranet.who.int/prequal/).
- Ensuring sufficient volume and specimen quality is important to obtain accurate results.
- Safe waste disposal of used test consumables needs to be planned in advance to minimize environmental risk.
- Trace positive results on respiratory samples may present false-positive results for TB disease (M. tb. non-viable but DNA detected) in those that are HIV negative or not at risk for HIV, and those with a prior history of TB and an end of treatment within the last 5 years.
- For tests that do not have integrated rifampicin-resistance detection as an all-in-one test, reflex testing for resistance should be performed at the same time for all TB-positive patients to support universal access to DST for rifampicin, at a minimum, and to reduce the risk of loss to follow-up.
- In settings with a very low prevalence of rifampicin resistance⁷ , i.e. less than 2%, a positive test result for rifampicin resistance may represent a false positive result, and indicate a need for further testing with an alternative method or, at a minimum, repeat testing.
- If rifampicin resistance is detected, further resistance testing for fluoroquinolones and bedaquiline is essential to guide selection of a shorter multidrug-resistant TB or rifampicin-resistant TB (MDR/RR-TB) treatment regimen.
- Use of a higher volume of CSF (≥6 mL) with concentration, where possible, is encouraged to increase the sensitivity of LC-aNAATs.
Monitoring and evaluation
- Track unsuccessful and indeterminate test result rates for currently recommended products and new products to be introduced in this class.
- Monitor the proportion of trace results from paucibacillary samples (e.g. CSF), including those that are culture-positive or culture-negative.
- Undertake surveillance to monitor the frequency of mutations (e.g. I491F mutation) outside of a rpoB rifampicin-resistance determining region (RRDR) over time.
- Monitor the proportion of people with bacteriologically confirmed TB without a rifampicin-resistance result or further recommended drug susceptibility reflex testing over time.
Research priorities
- Review the field performance of the current technologies used in routine practice (programmatic settings).
- Conduct operational research to ensure that tests are used optimally in terms of both clinical efficiency and cost efficiency in intended settings.
- Evaluate the impact of LC-aNAAT testing on patient-important outcomes (cure, mortality, time to diagnosis and time to start of treatment).
- Evaluate the strengths, weaknesses and cost differences of different LC-aNAAT products to inform country selection.
- Evaluate the different classes of tests, including LC-aNAATs, to determine which classes or testing strategies yield superior diagnostic accuracy, cost–effectiveness and impact on equity and acceptability.
- Evaluate the impact on incremental accuracy and case detection and the cost–effectiveness of alternative sample types that are easier to collect.
- Evaluate the individual product performance with different paediatric and extrapulmonary TB sample types.
- Develop new tools that are rapid, affordable, feasible and acceptable to children and their parents.
- Optimize or develop tests or simple pre-step sample handling procedures for extrapulmonary TB.
- Identify an improved reference standard that accurately defines TB disease in children, paucibacillary specimens, and people who cannot produce sputum, because the sensitivity of all available diagnostics is suboptimal.
- Develop and apply standardized methods for cost–effectiveness and economic studies, to limit variability.
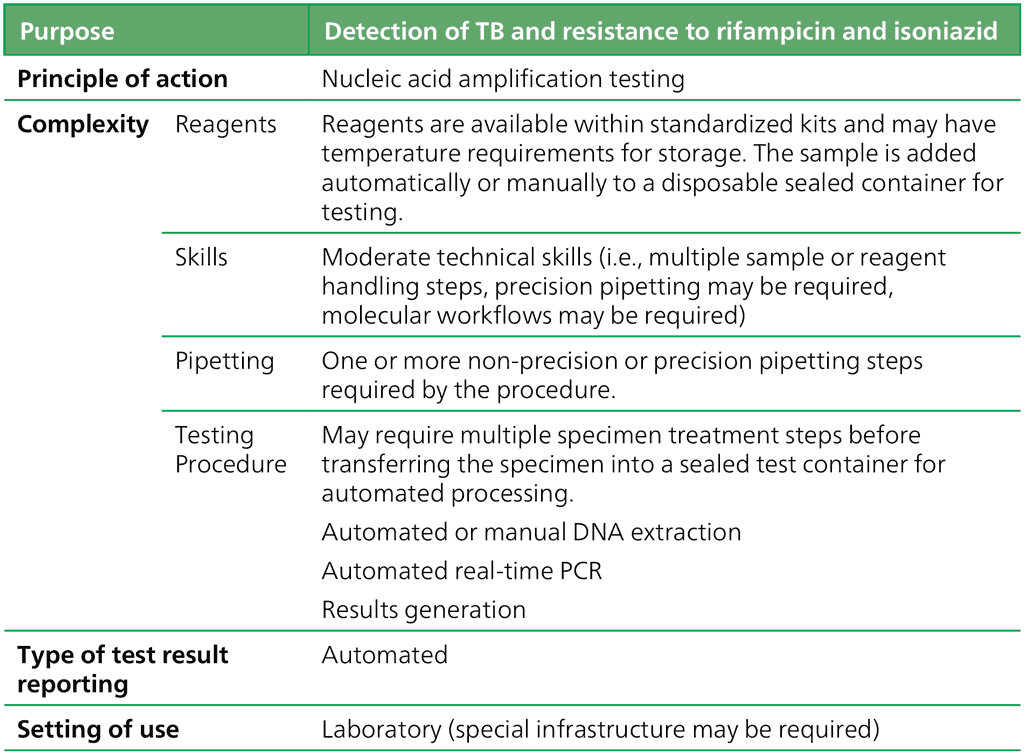
2.1.2 Moderate complexity automated NAATs for detection of TB and resistance to rifampicin and isoniazid
Rapid detection of TB and rifampicin resistance is increasingly available as new technologies are developed and adopted by countries. However, what has also emerged is the relatively high burden of isoniazid-resistant, rifampicin-susceptible TB that is often undiagnosed. Globally, isoniazid-resistant, rifampicin-susceptible TB is estimated to occur in 13.1% (95% CI: 9.9–16.9%) of new cases and 17.4% (95% CI: 0.5–54.0%) of previously treated cases (1).
A new class of technologies has come to market with the potential to address this gap. Several manufacturers have developed moderate complexity automated NAATs for detection of TB and resistance to rifampicin and isoniazid on high throughput platforms for use in laboratories. The tests belonging to this class are faster and less complex to perform than phenotypic culture-based drug susceptibility testing (DST) and line probe assays (LPA). They have the advantage of being largely automated following the sample preparation step. Moderate complexity automated NAATs may be used as an initial test for detection of TB and resistance to both first-line TB drugs simultaneously (rifampicin and isoniazid). They offer the potential for the rapid provision of accurate results (important to patients) and for testing efficiency where high volumes of tests are required daily (important to programmes). Hence, these technologies are suited to areas with a high population density and rapid sample referral systems.
Table 2.1.2.1 Class criteria for MC-aNAATs
Recommendation
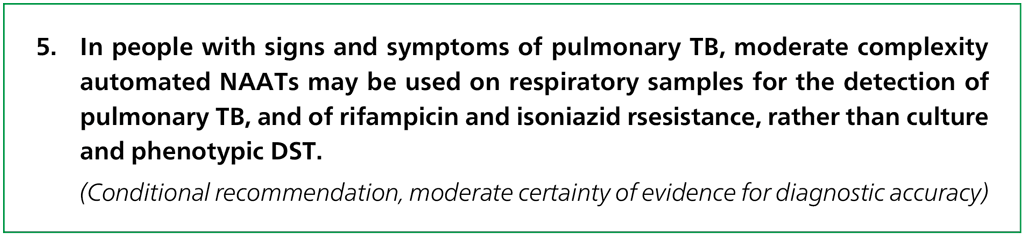
There are several subgroups to be considered for this recommendation:
- The recommendation is based on evidence of diagnostic accuracy in respiratory samples of adults with signs and symptoms of pulmonary TB.
- The recommendation applies to people living with HIV (studies included a varying proportion of such individuals); performance on smear-negative samples was reviewed but was only available for TB detection, not for rifampicin and isoniazid resistance, and data stratified by HIV status were not available.
- The recommendation applies to adolescents and children based on the generalization of data from adults; an increased rate of indeterminate results may be found with paucibacillary TB disease in children.
- The review did not consider extrapolation of the finding for use in people with extrapulmonary TB and testing on non-sputum samples because data on diagnostic accuracy of technologies in the class for non-sputum samples were limited.
Justification and evidence
The WHO Global TB Programme initiated an update of the current guidelines and commissioned a systematic review on the use of moderate complexity automated NAATs for detection of TB and resistance to rifampicin and isoniazid in people with signs and symptoms of TB.
Three PICO questions were designed to form the basis for the evidence search, retrieval and analysis:
- Should moderate complexity automated NAATs be used on respiratory samples in people with signs and symptoms of pulmonary TB for detection of pulmonary TB, as compared with culture?
- Should moderate complexity automated NAATs be used on respiratory samples in people with signs and symptoms of pulmonary TB for detection of resistance to rifampicin, as compared with culture-based phenotypic DST?
- Should moderate complexity automated NAATs be used on respiratory samples in people with signs and symptoms of pulmonary TB for detection of resistance to isoniazid, as compared with culture-based phenotypic DST?
A comprehensive search of the following databases (PubMed, Embase, BIOSIS, Web of Science, LILACS and Cochrane) for relevant citations was performed. The search was restricted to the period January 2009 to July 2020. Reference lists from included studies were also searched. No language restriction was applied. Because there were few studies for the selected index tests, the diagnostic companies were contacted for reports of their internal validation data. Studies were also included from the WHO public call for submission of data. Mycobacterial culture was used as the reference standard for evaluation of Mtb detection. Resistance detection was compared with a phenotypic DST reference standard and a composite reference standard (that combines phenotypic and genotypic DST results) in studies where both had been performed.
Bivariate random-effects meta-analyses were performed using Stata software, to obtain pooled sensitivity and specificity estimates with 95% CIs for rifampicin resistance, isoniazid resistance and Mtb detection. Where only a limited number of studies were available, descriptive analyses were conducted.
For meta-analysis, studies were first meta-analysed separately for each test. Studies from all the tests were then used to obtain a pooled estimate for all technologies.
To decide whether pooling of all the tests would give meaningful estimates, various criteria for pooling were developed and agreed upon by the GDG panel before they were applied. Data were also evaluated and visualized using head-to-head comparisons of the tests with Xpert® MTB/RIF or any other WHO-recommended test.
Data for all the index platforms were only pooled to answer PICO questions if they met the preconditions given in Table 2.1.2.2 and fulfilled either Condition 1 or Condition 2.
Table 2.1.2.2 Criteria for pooling studies on moderate complexity automated NAATs
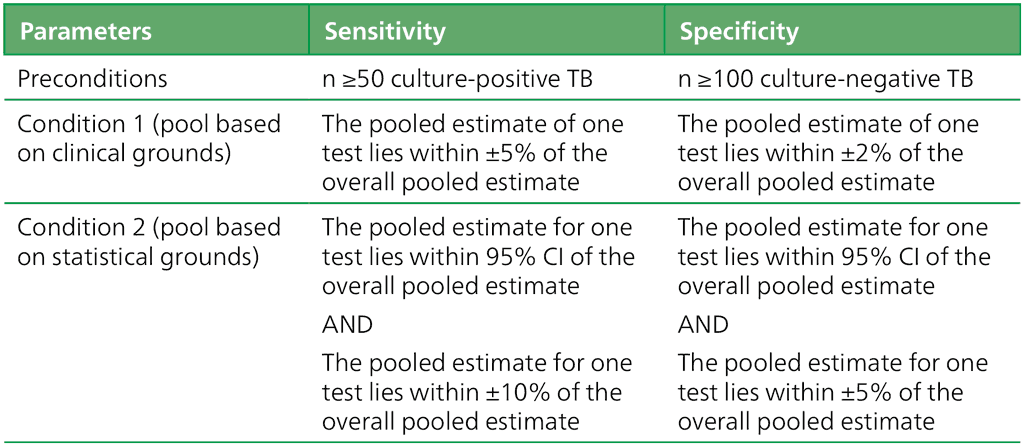
CI: confidence interval; n: number; NAAT: nucleic acid amplification test; TB: tuberculosis.
Data synthesis was structured around the three preset PICO questions, as outlined below. Three web annexes⁸ give additional information, as follows:
- details of studies included in the current analysis (Web Annex 1.3: Moderate complexity automated NAATs;
- a summary of the results and details of the evidence quality assessment (Web Annex 2.3: Moderate complexity automated NAATs); and
- a summary of the GDG panel judgements (Web Annex 3.3: Moderate complexity automated NAATs).
PICO 1: Should moderate complexity automated NAATs be used on respiratory samples in people with signs and symptoms of pulmonary TB for detection of pulmonary TB, as compared with culture?
A total of 29 studies with 13 852 specimens provided data for evaluating TB detection from the five index tests (Fig. 2.1.2.1). Of these 29 studies, 12 were conducted on the Abbott RealTime MTB test, six on FluoroType MTB, four on FluoroType MTBDR, five on BD MAX and two on the cobas MTB test. The reference standard for each of these studies for TB detection was mycobacterial culture.
Of the 29 studies, 16 (55%) had high or unclear risk of bias because they tested specimens before inclusion in the study, used convenience sampling or did not report the method of participant selection. Thus, the evidence was downgraded one level for risk of bias. Overall, the certainty of the evidence was moderate for sensitivity and high for specificity.
Fig. 2.1.2.1 Forest plot of included studies for TB detection with culture as the reference standard
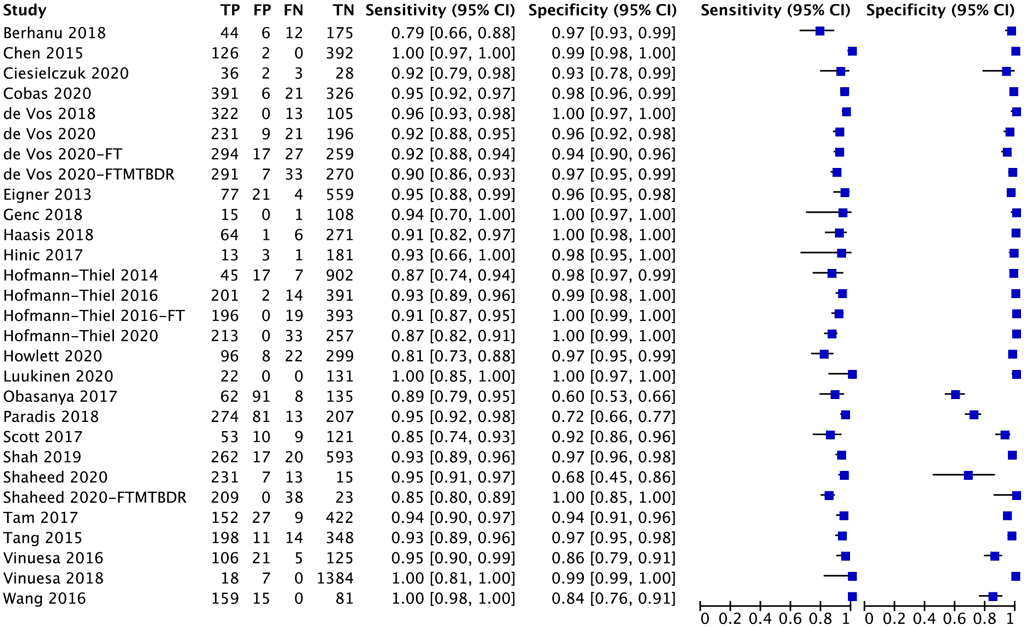
CI: confidence interval; FN: false negative; FP: false positive; TB: tuberculosis; TN: true negative; TP: true positive.
The overall sensitivity in these 29 studies ranged from 79% to 100%, and the specificity from 60% to 100%. The pooled sensitivity was 93.0% (95% CI: 90.9–94.7%) and the pooled specificity was 97.7% (95% CI: 95.6–98.8%).
PICO 2: Should moderate complexity automated NAATs be used on respiratory samples in people with signs and symptoms of pulmonary TB for detection of resistance to rifampicin, as compared with culture-based phenotypic DST?
A total of 18 studies with 2874 specimens provided data for resistance testing of rifampicin using moderate complexity automated NAATs (Fig. 2.1.2.2). Of these 18 studies, nine were conducted on the Abbott RealTime RIF/INH test, three on FluoroType MTBDR, four on BD MAX and two on the cobas RIF/INH test. The reference standard for each of these studies for resistance detection was phenotypic DST, using a composite reference standard with both phenotypic DST and sequencing results.
Eight (44%) of the 18 studies had high or unclear risk of bias because they did not report participant selection or tested specimens before inclusion in the study.
Fig. 2.1.2.2 Forest plot of included studies for rifampicin resistance detection with phenotypic DST as the reference standard
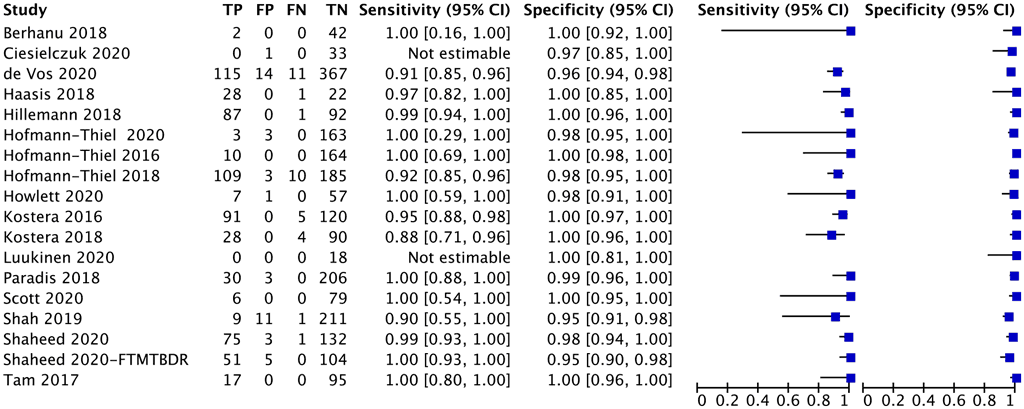
CI: confidence interval; DST: drug susceptibility testing; FN: false negative; FP: false positive; TB: tuberculosis; TN: true negative; TP: true positive.
The overall sensitivity for rifampicin resistance in these 18 studies ranged from 88% to 100% and the specificity from 98% to 100%. The pooled sensitivity was 96.7% (95% CI: 93.1– 98.4%) and the pooled specificity was 98.9% (95% CI: 97.5–99.5%).
In determining rifampicin resistance, the results from genetic sequencing (genotypic DST) were obtained where possible, and a composite reference standard was developed that combined the results from phenotypic and genotypic DST. For rifampicin resistance detection, the diagnostic test accuracy of moderate complexity automated NAATs was similar for phenotypic DST and the composite reference standard.
PICO 3: Should moderate complexity automated NAATs be used on respiratory samples in people with signs and symptoms of pulmonary TB for detection of resistance to isoniazid, as compared with culture-based phenotypic DST?
A total of 18 studies with 1758 specimens provided data for resistance testing of isoniazid using moderate complexity automated NAATs (Fig. 2.1.2.3). Of these 18 studies, nine were conducted on the Abbott RealTime RIF/INH test, three on FluoroType MTBDR, four on BD MAX and two on the cobas MTB-RIF/INH test. The reference standard for each of these studies for resistance detection was phenotypic DST, and a composite reference standard with both phenotypic DST and sequencing results.
Eight (44%) of the 18 studies had high or unclear risk of bias, because participant selection was not reported or prior testing was done on the included specimens.
Fig. 2.1.2.3 Forest plot of included studies for isoniazid resistance detection with phenotypic DST as the reference standard
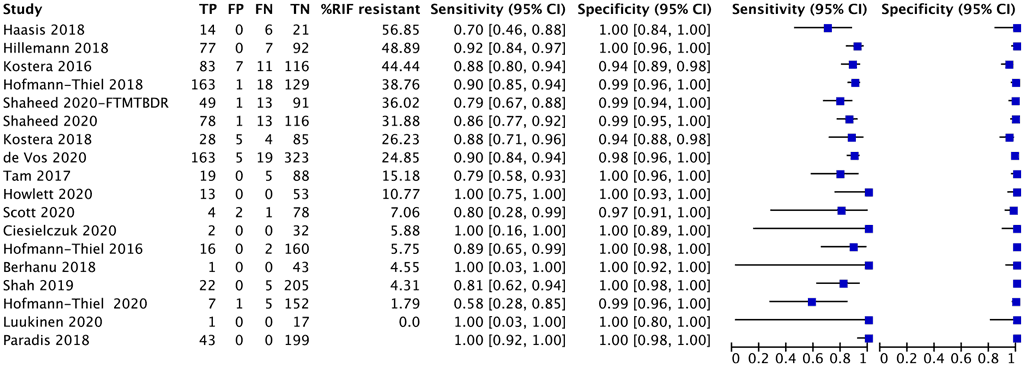
CI: confidence interval; DST: drug susceptibility testing; FN: false negative; FP: false positive; RIF: rifampicin; TB: tuberculosis; TN: true negative; TP: true positive.
The overall sensitivity for isoniazid resistance in these 18 studies ranged from 58% to 100% and the specificity from 94% to 100%. The pooled sensitivity was 86.4% (95% CI: 82.1– 89.8%) and the pooled specificity was 99.8% (95% CI: 98.3–99.8%).
In determining isoniazid resistance, the results from genetic sequencing (genotypic DST) were obtained where possible, and a composite reference standard was developed that combined the results from phenotypic and genotypic DST. For detecting isoniazid resistance, the diagnostic test accuracy of phenotypic DST was similar to that of the composite reference standard.
Cost–effectiveness analysis
This section answers the following additional question:
What is the comparative cost, affordability and cost–effectiveness of implementation of moderate complexity automated NAATs?
A systematic review was conducted, focusing on economic evaluations of moderate complexity automated NAATs. Four online databases (Embase, Medline, Web of Science and Scopus) were searched for new studies published from 1 January 2010 through 17 September 2020. The citations of all eligible articles, guidelines and reviews were reviewed for additional studies. Experts and test manufacturers were also contacted to identify any additional unpublished studies.
The objective of the review was to summarize current economic evidence and further understand the costs, cost–effectiveness and affordability of moderate complexity automated NAATs.
Several commercially available tests were included as eligible tests in the moderate complexity automated NAATs category; however, no published studies were identified assessing the costs or cost–effectiveness of any of those tests. One unpublished study comparing available data on two technologies from moderate complexity automated NAATs class was identified, and the data from that study are described below.
Unpublished data from FIND was provided through direct communication. This costing-only study used time and motion studies combined with a bottom-up, ingredients-based approach to estimate the unit test cost for the two selected technologies.⁹ Time and motion studies were conducted at a reference-level laboratory in South Africa. Several important simplifying assumptions were made that may limit the generalizability of the results; for example, 50% of laboratory operations dedicated to TB, a minimum daily throughput of 24 samples or the equivalent of one BD MAX run (24 tests/run), equipment costs fixed at US$ 100 000 for both platforms, a 5% annual maintenance cost, and the standard 3% discount rate and 10 years expected useful life years.
Additional literature searches conducted to look for economic data using similar platforms from non-TB disease areas identified three additional studies from HIV and hepatitis C virus (HCV) with limited cost data: one (5) using Abbott RealTime HIV and two on HCV (6,7). Data were limited to cost per unit test kit and are not transferrable to test kit costs for the tests being considered in this review.
How large are the resource requirements (costs)?
Available unit test costs for two moderate complexity automated NAATs ranged from US$ 18.52 (US$ 13.79–40.70) and US$ 15.37 (US$ 9.61–37.40), with one study reporting cheaper per-test kit costs and higher operational costs associated with laboratory processing time. Equipment costs were strong drivers of cost variation and will vary across laboratory networks and operations. If equipment can be optimally placed or multiplexed to ensure high testing volume, the per-test cost can be minimized.
In one-way sensitivity analyses, annual testing volumes varied from fewer than 5000 tests/year to more than 25 000 tests/year. Per-test cost was highly sensitive to testing volume when fewer than 5000 tests were conducted per year; however, unit test costs begin to stabilize between 5000 and 10 000 tests/year, and above 10 000 tests/year, unit cost estimate was robust. When equipment can be multiplexed and used at capacity, per-test cost can be minimized.
What is the certainty of the evidence of resource requirements (costs)?
Available per-test cost data were unpublished but did include overheads, equipment, building, staff and consumable costs; however, complete quality assessment of the study was not possible. Test cost will vary according to testing volume and laboratory operations. There is limited evidence to assess the important variability across sites, countries and implementation approaches.
Does the cost–effectiveness of the intervention favour the intervention or the comparison?
No studies were identified that assessed cost–effectiveness for any of the moderate complexity automated NAATs, and extrapolation was not appropriate given differences in standard of care, care cascades and associated costs, operational conditions, testing volume and diagnostic accuracy. Implementation considerations (e.g. test placement, laboratory network and ability of the programme to initiate treatment quickly) are all likely to affect unit test cost and cost– effectiveness. Economic modelling is needed across various settings to understand the range of cost–effectiveness profiles of moderate complexity automated NAATs, and how they are likely to vary under different operational criteria.
Additional details on economic evidence synthesis and analysis are provided in Web Annex B.12: Systematic literature review of economic evidence for NAATs to detect TB and DR-TB in adults and children.
User perspective
This section answers the following questions about key informants’ views and perspectives on the use of moderate complexity automated NAATs:
- Is there important uncertainty about or variability in how much end-users value the main outcomes?
- What would be the impact on health equity?
- Is the intervention acceptable to key stakeholders?
- Is the intervention feasible to implement?
User perspectives on the value, feasibility, usability and acceptability of diagnostic technologies are important in the implementation of such technologies. If the perspectives of laboratory personnel, clinicians, patients and TB programme personnel are not considered, the technologies risk being inaccessible to and underused by those for whom they are intended.
To address questions related to user perspective, two activities were undertaken:
- A systematic review of evidence on user perspectives and experiences with NAATs for detection of TB and TB drug resistance (moderate and low complexity automated assays, and high complexity hybridization-based assays) was undertaken from July to November 2020.
- A total of 14 semi-structured interviews with clinicians, programme officers, laboratory staff and patient advocates were conducted in India, Moldova and South Africa from October to November 2020.
The findings from these activities are discussed below.
Systematic review
A total of 27 studies were identified that met inclusion criteria, of which 21 were sampled for inclusion in the analysis. All of the sampled studies were published between 2012 and 2020. Of the 21 included studies, 18 were located in high TB burden countries: six in India, four in South Africa, two each in Kenya and Uganda, and one each in Brazil, Cambodia, Myanmar and Viet Nam. One study covered projects in nine countries (Bangladesh, Cambodia, Democratic Republic of the Congo, Kenya, Malawi, Moldova, Mozambique, Nepal and Pakistan). In addition, there was one study located in Eswatini, one in Mongolia and one in Nepal. All studies focused on Xpert MTB/RIF, except for one that focused on Xpert MTB/RIF Ultra (Xpert Ultra).
A summary of the core characteristics of studies included in this review is presented in a study characteristics table in Web Annex B.13: User perspectives on NAATs to detect TB and DR-TB: results from qualitative evidence synthesis: systematic review.
Interviews
The aim of the interviews was to understand participants’ experiences of using the various technologies (i.e. NAATs for detection of TB and TB drug resistance) and their general TB diagnostic experiences. The three countries – India, Moldova and South Africa – were selected based on them being on WHO’s list of 30 high MDR-TB burden countries (1) and that index tests have been used to some extent in research contexts within these countries. Due to the short time frame, participants were purposively sampled and approached based on convenience through personal contacts and colleagues.
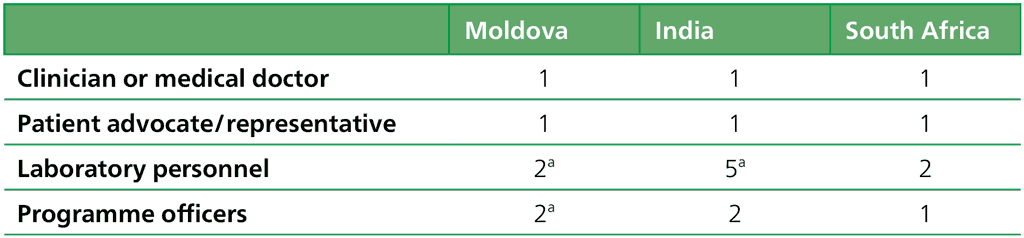
An overview of the participants is given in Table 2.1.2.3 To mask the identity of study participants they were coded by their country (Moldova [M], India [I] or South Africa [S]), their profession (clinician or medical doctor [M], patient advocate/representative [R], laboratory personnel [L] or programme officers [P]) and a number.
Table 2.1.2.3 Overview of participants for the end-users’ interviews
a Participants were interviewed as a group.
Interviews were conducted using Zoom, Skype or phone. Topics discussed included:
- current approach to diagnosing TB, MDR-TB and extensively drug-resistant TB (XDR-TB), including specific challenges;
- experiences with using molecular TB diagnostics and the index tests specifically, including details on steps taken in the diagnostic process;
- experiences with determining eligibility and treatment initiation, and challenges and benefits of using the index tests;
- overall usefulness of the index tests;
- the feasibility of implementing the index tests;
- the potential impact of the index tests on health equity; and
- how the potential impact of the index tests relates to current policy context.
Several important limitations of this approach were noted. Only a few participants were interviewed per country. Owing to the use of Zoom, Skype or phone for interviews, it was not possible to triangulate interview data with other evidence commonly collected through ethnographic approaches (e.g. multiple interviews and informal conversations at the same facility, observations or site visits). In addition, only some of the participants had personal experience with one or all of the index tests, and those participants who did have experience with the tests had used them in research settings rather than for routine practice.
More details on these interviews are given in Web Annex B.14: User perspectives on nucleic acid amplification tests for tuberculosis and tuberculosis drug resistance: Interviews study.
Findings of the review and interviews
The main findings of the systematic review and interviews are given below. Where information is from the review, a level of confidence in the quality evidence synthesis (QES) is given; where it is from interviews, this is indicated with ‘Interviews’.
Is there important uncertainty about or variability in how much end-users value the main outcomes?
- Patients in high burden TB settings value:
- getting an accurate diagnosis and reaching diagnostic closure (finally knowing “what is wrong with me”);
- avoiding diagnostic delays because they exacerbate existing financial hardships and emotional and physical suffering, and make patients feel guilty for infecting others (especially children);
- having accessible facilities; and
- reducing diagnosis-associated costs (e.g. travel, missing work) as important outcomes of the diagnostic.
QES: moderate confidence
- Moderate complexity automated NAATs meet several preferences and values of clinicians and laboratory staff, in that they:
- are faster than culture-based phenotypic DST (similar to LPA or cartridge-based tests);
- have the advantage of being automated (unlike LPA);
- provide additional clinically relevant drug-resistance information such as high versus low resistance (unlike the current Xpert MTB/RIF cartridge).
Interviews
What would be the impact on health equity?
- Various factors – for example, lengthy diagnostic delays, underuse of diagnostics, lack of TB diagnostic facilities at lower levels and too many eligibility restrictions – hamper access to prompt and accurate testing and treatment, particularly for vulnerable groups.
QES: high confidence - Staff and managers voiced concerns about:
- sustainability of funding and maintenance;
- complex conflicts of interest between donors and implementers; and
- the strategic and equitable use of resources, which negatively affects creating equitable access to cartridge-based diagnostics.
QES: high confidence
- Access to clear and comprehensible information for TB patients on what TB diagnostics are available to them and how to interpret results is a vital component of equity, and lack of such access represents an important barrier for patients.
Interviews - New treatment options need to be matched with new diagnostics. It is important to improve access to treatment based on new diagnostics and to improve access to diagnostics for new treatment options.
Interviews - The speed at which WHO guidelines are changing does not match the speed at which many country programmes are able to implement the guidelines. This translates into differential access to new TB diagnostics and treatment:
- between countries (i.e. between those that can and cannot quickly keep up with the rapidly changing TB diagnostic environment); and
- within countries (i.e. between patients who can and cannot afford the private health system that is better equipped to quickly adopt new diagnostics and policies).
Interviews
- The identified challenges with the use of NAATs for detection of TB and DR-TB, and accumulated delays, risk compromising the added value as identified by the users, ultimately leading to underuse. The challenges also hamper access to prompt and accurate testing and treatment, particularly for vulnerable groups.
QES: high confidence
Is the intervention acceptable to key stakeholders?
- Patients can be reluctant to test for TB or MDR-TB because of:
- stigma related to MDR-TB or having interrupted treatment in the past;
- fears of side-effects;
- failure to recognize symptoms;
- inability to produce sputum; and
- cost, distance and travel concerns related to (repeat) clinic visits.
QES: high confidence
- Health workers can be reluctant to test for TB or MDR-TB because of:
- TB-associated stigma and consequences for their patients;
- fear of acquiring TB;
- fear from supervisors when reclassifying patients already on TB treatment who turn out to be misclassified;
- fear of side-effects of drugs in children; and
- community awareness of disease manifestations in children.
QES: high confidence
- In relation to the acceptability of moderate complexity automated NAATs:
- the automation of this class of technologies, which recognizes the high workload of laboratory staff, improves their acceptability;
- in terms of the physical size of the platform and how it fits into the laboratory space and workflow, a smaller footprint may be more acceptable; and
- the number of samples run on the system is acceptable provided that the platform is placed within a laboratory that receives a sufficient sample load to run the system.
Interviews
Is the intervention feasible to implement?
- The feasibility of all diagnostic technologies is challenged if there is an accumulation of diagnostic delays or underuse (or both) at every step in the process, mainly because of health system factors such as:
- non-adherence to testing algorithms, testing for TB or MDR-TB late in the process, empirical treatment, false negatives due to technology failure, large sample volumes and staff shortages, poor or delayed sample transport and sample quality, poor or delayed communication of results, delays in scheduling follow-up visits and recalling patients, and inconsistent recording of results;
- lack of sufficient resources and maintenance (i.e. stock-outs; unreliable logistics; lack of funding, electricity, space, air conditioners and sputum containers; dusty environment; and delayed or absent local repair option);
- inefficient or unclear workflows and patient flows (e.g. inefficient organizational processes, poor links between providers, and unclear follow-up mechanisms or information on where patients need to go); and
- lack of data-driven and inclusive national implementation processes.
QES: high confidence
- The feasibility of moderate complexity automated NAATs is also challenged by:
- how or whether the platform fits into the physical space of the laboratory (considering bench size and weight of the platform) and sample workflow;
- a poorly functioning sample transport system that affects the quality of samples; and
- the need to ensure that clinicians and laboratory staff have time to communicate effectively regarding diagnostic results if the platform is centralized, while also ensuring that the laboratory location is central enough to receive adequate numbers of samples to make the machine worth running.
Interviews
- Implementation of new diagnostics must be accompanied by training for clinicians to help them interpret results from new molecular tests and understand how this information is translated into prompt and proper patient management. In the past, with the introduction of Xpert MTB/RIF, this has been a challenge.
QES: high confidence and interviews - Introduction of new diagnostics must be accompanied by guidelines and algorithms that support clinicians and laboratories in communicating with each other, such that they can discuss discordant results and interpret laboratory results in the context of drug availability, patient history and patient progress on a current drug regimen.
Interviews
Implementation considerations
Factors to consider when implementing moderate complexity automated NAATs for detection of TB and resistance to rifampicin and isoniazid are as follows:
- local epidemiological data on resistance prevalence should guide local testing algorithms, whereas pretest probability is important for the clinical interpretation of test results;
- the cost of a test varies depending on parameters such as the number of samples in a batch and the staff time required; therefore, a local costing exercise should be performed;
- low, moderate and high complexity tests have successive increase in technical competency needs (qualifications and skills) and staff time, which affects planning and budgeting;
- availability and timeliness of local support services and maintenance should be considered when selecting a provider;
- laboratory accreditation and compliance with a robust quality management system (including appropriate quality control) are essential for sustained service excellence and trust;
- training of both laboratory and clinical staff is needed to ensure effective delivery of services and clinical impact;
- use of connectivity solutions for communication of results is encouraged, to improve efficiency of service delivery and reduce time to treatment initiation;
- moderate complexity automated NAATs may already be used programmatically for other diseases – for example, severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), HIV and antimicrobial resistance (AMR) – which could potentially facilitate implementation of TB testing on shared platforms;
- implementation of moderate complexity automated NAATs requires laboratories with the required infrastructure, space and efficient sample referral systems;
- although these are automated tests, well-trained skilled staff are needed to set up assays and complete maintenance requirements; and
- implementation of these tests should be context specific; thus, it should take into account access issues, especially in remote areas, where less centralized WHO-recommended technologies may be more appropriate.
Research priorities
Research priorities for moderate complexity automated NAATs for detection of TB and resistance to rifampicin and isoniazid are as follows:
- diagnostic accuracy in specific patient populations (e.g. children, people living with HIV, and patients with signs and symptoms of extrapulmonary TB) and in non-sputum samples;
- impact of diagnostic technologies on clinical decision-making and outcomes that are important to patients (e.g. cure, mortality, time to diagnosis and time to start treatment) in all patient populations;
- impact of specific mutations on treatment outcomes among people with DR-TB;
- use, integration and optimization of diagnostic technologies in the overall landscape of testing and care, as well as diagnostic pathways and algorithms;
- economic studies evaluating the costs, cost–effectiveness and cost–benefit of different diagnostic technologies;
- qualitative studies evaluating equity, acceptability, feasibility and end-user values of different diagnostic technologies;
- effect of non-actionable results (indeterminate, non-determinate or invalid) on diagnostic accuracy and outcomes that are important to patients;
- operational research on the advantages and disadvantages of individual technologies within the class of moderate complexity automated NAATs;
- effect of moderate complexity automated NAATs in fostering collaboration and integration between disease programmes; and
- the potential utility of detecting katG resistance to identify MDR-TB clones that may be missed because they do not have an RRDR mutation (e.g. the Eswatini MDR-TB clone, which has both the katG S315T and the non-RRDR rpoB I491F mutation).
5 Having a positive result of a test, examination or other procedure used to distinguish people with a high likelihood of having TB disease from people who are highly unlikely to have TB. At present, the following tests are WHO-recommended as the screening tests: chest radiography (chest X-ray; CXR) with or without computer-aided detection (CAD), C-reactive protein (CRP) in people living with HIV, and molecular WHO-recommended rapid diagnostic test for TB (mWRD) (https://www.who.int/publications/i/item/9789240022676).
6 Data were not pooled due to the limited number of studies.
7 The 2% prevalence was used as the lowest one in evidence synthesis and analysis to inform GDG meeting. At this prevalence level the number of false-positive results amounted to 19 out of 1000 eligible patients tested and equalized number of truepositive results.
8 A complete list of web annexes is provided at pp 172–173.
9 Data courtesy of H Sohn and W Stevens at FIND (unpublished).

 Feedback
Feedback